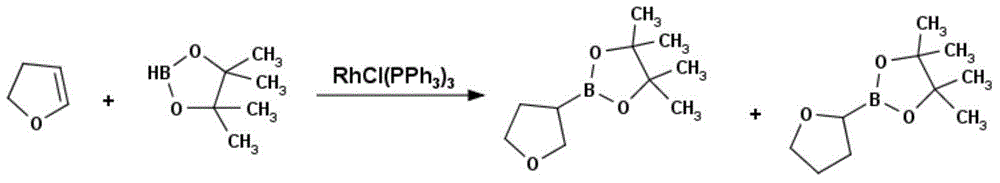
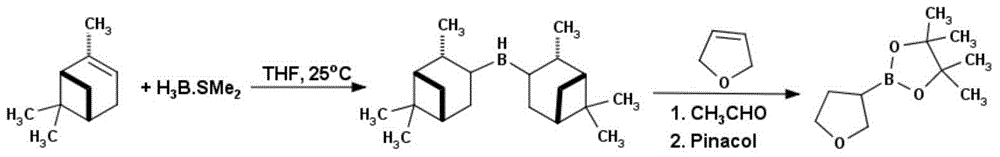
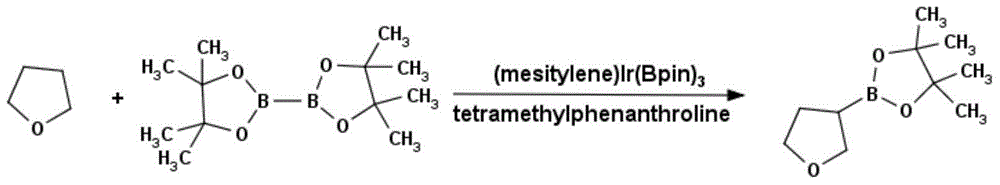Method for preparing tetrahydrofuran-3-boric acid pinacol ester
A technology of tetrahydrofuran and dihydrofuran, which is applied in the field of organic chemical synthesis, can solve the problems of expensive raw material pinacol borane and transition metal catalyst, restrict mass production of target products, and cannot realize enlarged production, etc. The effect of high yield and low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 1L three-necked reaction flask, add D-α-pinene (50g, 0.37mol) and 200mL of anhydrous tetrahydrofuran. 0.17mol, 10M in dimethylsulfide), after the addition, the temperature was raised to room temperature, reacted for 4h, and a white solid was generated, then the reaction solution was cooled to -40°C, and 2,5-dihydrofuran (12g, 0.17mol ), after the addition was completed, after rising to room temperature, reacted for 12h, added anhydrous acetaldehyde (75g, 1.7mol), reacted at room temperature for 6h, removed excess anhydrous acetaldehyde under reduced pressure, and then pinacol (20.1g, 0.17 mol), reacted at room temperature for 12h, and evaporated the solvent under reduced pressure with a rotary evaporator, and collected 107-109°C / 1mmHg fractions by distillation under reduced pressure to obtain 24.2g of a colorless liquid, which was tetrahydrofuran-3-boronic acid pinacol ester. rate of 72%. 1H NMR (CD 3 Cl): 3.99ppm, multimodal (1H); 3.80ppm, multimodal (1H); 3.70p...
Embodiment 2
[0029] In a 10L three-necked reaction flask, add D-α-pinene (2331.15g, 17.11mol) and 2.5L anhydrous tetrahydrofuran, under the protection of nitrogen, cool to 0 ° C, slowly dropwise add borane dimethyl sulfide complex ( 815mL, 8.15mol, 10M in dimethylsulfide), after the addition, the temperature was raised to room temperature, and reacted for 4h to generate a white solid, then the reaction solution was cooled to -40°C, and 2,5-dihydrofuran (571g, 8.15mol), the addition was completed, after rising to room temperature, reacted for 12h, added anhydrous acetaldehyde (3690g, 81.5mol), reacted at room temperature for 6h, removed excess anhydrous acetaldehyde under reduced pressure, and then pinacol (963g, 8.15mol), reacted at room temperature for 12h, evaporated the solvent under reduced pressure with a rotary evaporator, collected 107-109°C / 1mmHg fraction by distillation under reduced pressure, and obtained 1097.3g of a colorless liquid, which was tetrahydrofuran-3-boronic acid pina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com