Synthetic method of triphenoxyboroxine
A technology of triphenoxy boron and synthesis method, which is applied in the direction of chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, electrochemical generators, etc., which can solve unfavorable mass production and green environmental protection concepts, Problems such as large equipment investment and high energy consumption can achieve the effect of increasing reversible specific capacity, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
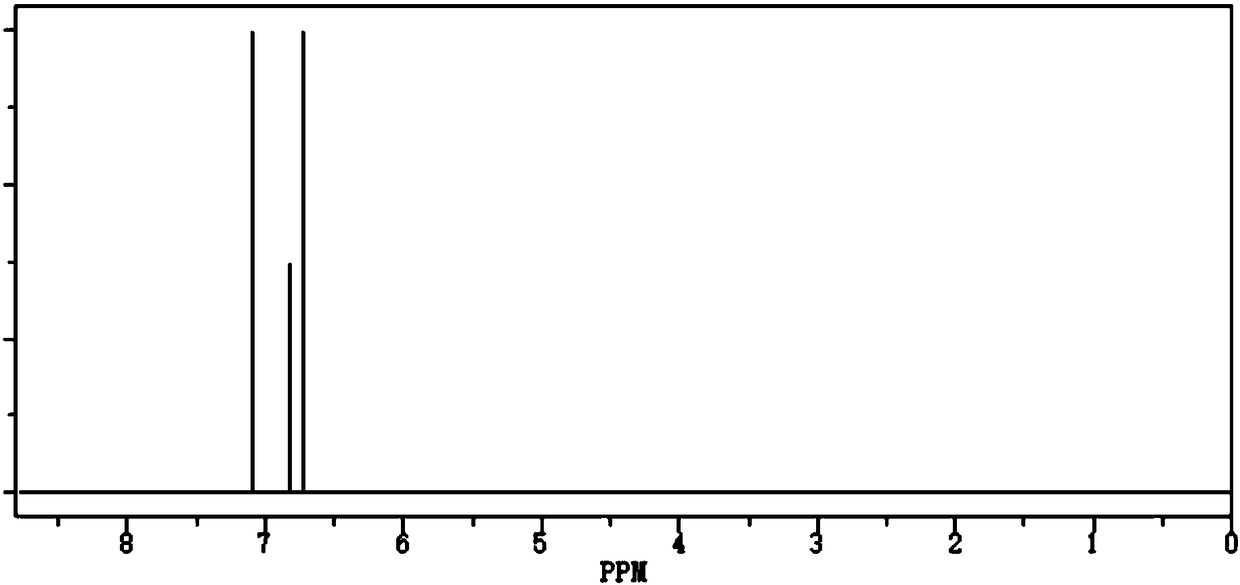
[0016] Add 1.02 mol of borane methyl sulfide into the Schlenk reaction flask, add 6 mol of THF to mix, then add 1 mol of phenol and 1 mol of water to it, heat and react at 70°C and 5 atm for 1 hour, then react at 80°C and pressure 120Pa for 30 minutes , and then reacted at 5 Pa and 100°C for 20 min, concentrated to dryness, and cooled to room temperature to obtain triphenoxyboroxane.
[0017] The density of the detected triphenoxyboroxane is 1.24g / cm 3 , boiling point 418°C 760mmHg, purity 99.58%, water content 38ppm, acid value 47ppm, yield 96.3%.
Embodiment 2
[0019] Add 1.03mol of borane methyl sulfide into the Schlenk reaction flask, add 8mol of THF to mix, then add 1mol of phenol and 1mol of water to it, heat the reaction at 80°C and 1 atm for 1.5h, and then react at 70°C and pressure of 80Pa 40min, and then reacted at 3Pa, 80°C for 30min, concentrated to dryness, and cooled to room temperature to obtain triphenoxyboroxane.
[0020] The density of the detected triphenoxyboroxane is 1.23g / cm 3 , boiling point 407°C 760mmHg, purity 99.56%, water content 40ppm, acid value 52ppm, yield 95.7%.
Embodiment 3
[0022] Add 1.02 mol of borane methyl sulfide into the Schlenk reaction flask, add 7 mol of THF to mix, then add 1 mol of phenol and 1 mol of water to it, heat and react at 75°C and 3 atm for 1.2h, then react at 75°C and pressure 100Pa 30min, then reacted at 4Pa, 90°C for 25min, concentrated and dried, and cooled to room temperature to obtain triphenoxyboroxane.
[0023] The density of the detected triphenoxyboroxane is 1.22g / cm 3 , boiling point 412°C 760mmHg, purity 99.78%, water content 30ppm, acid value 35ppm, yield 96.8%.
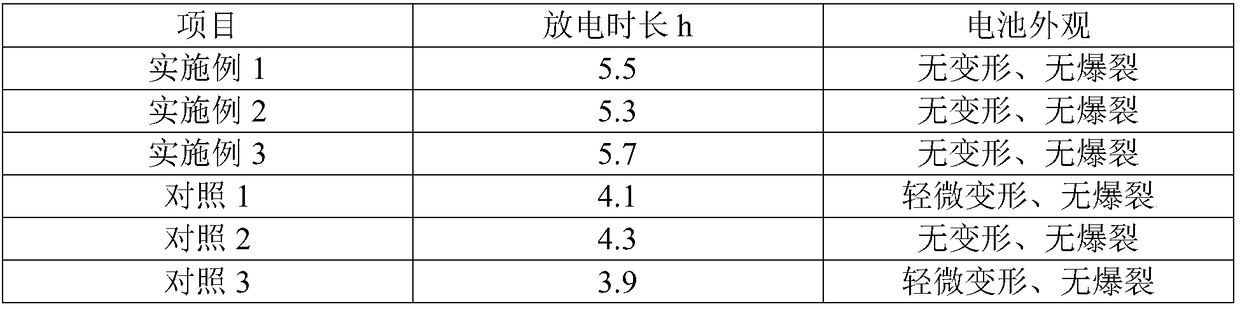
[0024] 2. Application test
[0025] Assembled batteries were tested for cycle performance. Lithium cobalt oxide was used as the positive electrode material, mesophase carbon microspheres were used as the negative electrode, aluminum foil and copper foil were used as current collectors for the positive and negative electrodes, and ceramic separators were used as the diaphragm to form a soft pack battery. After injecting the electrolyte, the The pouch b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com