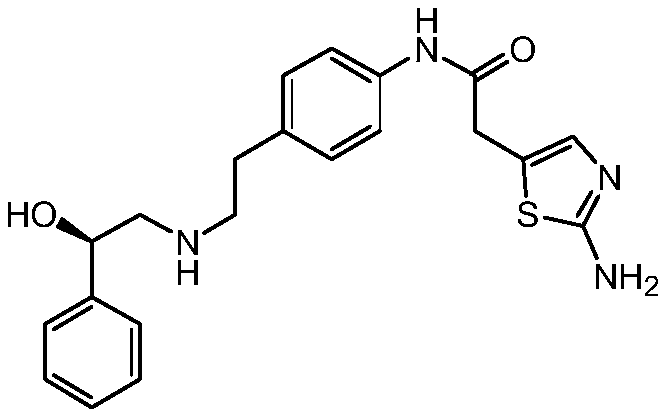
Synthesis method of Mirabegron
A synthetic method, aminomethyl technology, applied in the direction of organic chemistry, etc., can solve the problems of low mirabegron yield, high processing cost, and human health hazards, and achieve a green and environmentally friendly reaction process with fewer reaction steps and good The effect of market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
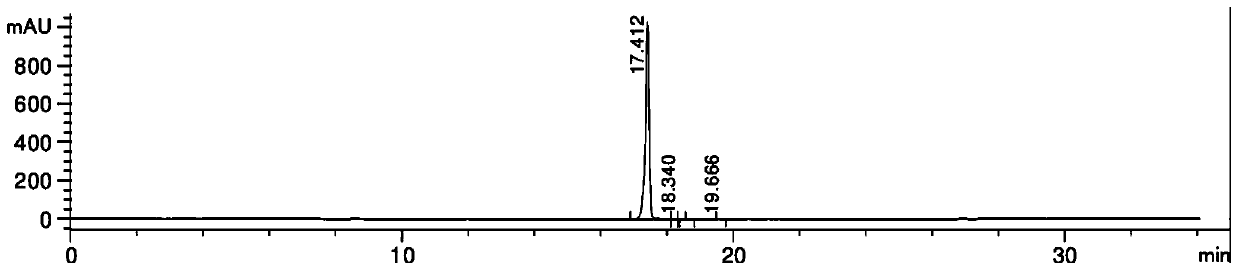
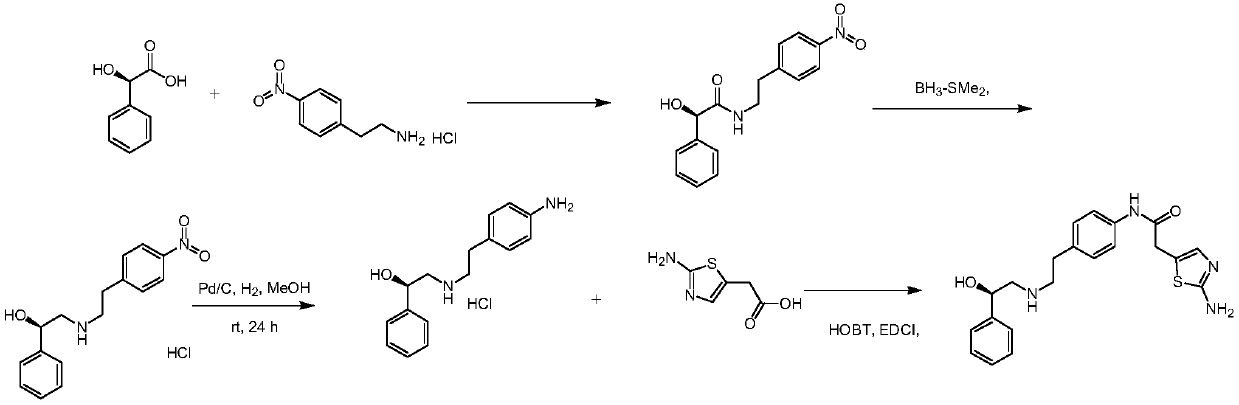
[0028] At room temperature, add methanol 300ml, R-2 (aminomethyl) benzyl alcohol 137 grams, 2-(2-aminothiazol-5-yl)-N-[4-(2-chloro-ethyl) -Phenyl]-acetamide 295g and 111g of triethylamine, heat the mixed solution to 60-65°C, stir and reflux for 8h, pump the reaction solution into 500g of water while it is hot, cool to 10-15°C, filter 380 g of off-white solid Mirabegron was obtained with a yield of 96% and a purity of 99.9%.
Embodiment 2
[0030] At room temperature, 300 ml of isopropanol, 137 grams of R-2 (aminomethyl) benzyl alcohol, 2-(2-aminothiazol-5-yl)-N-[4-(2-chloro-ethane base)-phenyl]-acetamide 295g and 111g of triethylamine, warm up the mixture to 60-65°C, stir and reflux for 8h, pump the reaction liquid into 500g of water while it is hot, and cool down to 10-15°C , and filtered to obtain 360g off-white solid mirabegron, with a yield of 91% and a purity of 99.47%.
Embodiment 3
[0032] At room temperature, add methanol 300ml, R-2 (aminomethyl) benzyl alcohol 137 grams, 2-(2-aminothiazol-5-yl)-N-[4-(2-chloro-ethyl) -Phenyl]-acetamide 295g and 141g of triethylamine, heat the mixture to 60-65°C, stir and reflux for 8h, pump the reaction liquid into 500g of water while it is hot, cool down to 10-15°C, filter 370 g of off-white solid mirabegron was obtained, with a yield of 94% and a purity of 98.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com