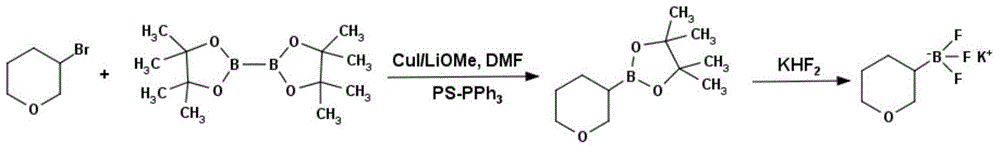
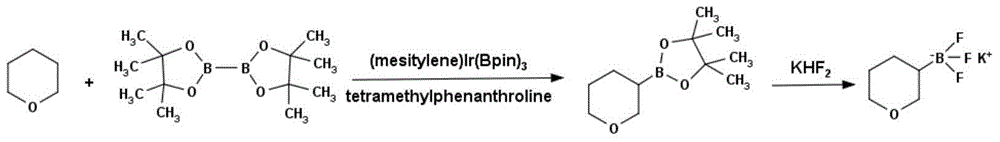
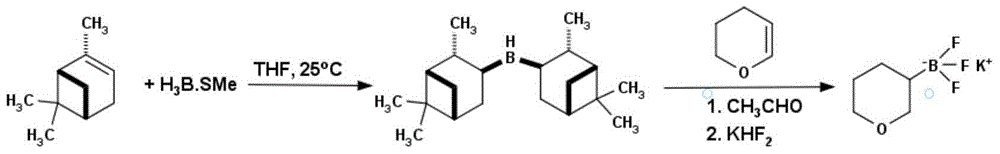
Method for preparing tetrahydropyran-3-potassium trifluoroborate
A technology of potassium trifluoroborate and tetrahydropyran, which is applied in the field of organic chemical synthesis, can solve the problems of high prices of transition metal catalysts and organic ligands, restrictions on the mass production of target products, and the impossibility of scale-up production, and achieve high yields High, low product cost, easy to store effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 1L three-necked reaction flask, add D-α-pinene (50g, 0.37mol) and 200mL of anhydrous tetrahydrofuran. 0.17mol, 10M in dimethyl sulfide), after the addition, the temperature was raised to room temperature, reacted for 4h, and a white solid was generated, then the reaction solution was cooled to -40°C, and 3,4-dihydropyran (14.3g, 0.17mol), after the addition was completed, after rising to room temperature, react for 12h, add anhydrous acetaldehyde (75g, 1.7mol), react at room temperature for 6h, remove excess anhydrous acetaldehyde under reduced pressure, and then add KHF 2 Saturated solution of (39.8g, 0.51mol), react at room temperature for 6h, evaporate the solvent under reduced pressure with a rotary evaporator, dry, extract the product with acetone, concentrate under reduced pressure, add ether to obtain 24.81g of a white solid, which is tetrahydropyridine Potassium pyran-3-trifluoroborate, yield 76%. 1HNMR (D 2 O): 3.76ppm, multimodal (2H); 3.12ppm, multimod...
Embodiment 2
[0029] In a 10L three-necked reaction flask, add D-α-pinene (2331.15g, 17.11mol) and 2L anhydrous tetrahydrofuran, under the protection of nitrogen, cool to 0°C, slowly add borane dimethyl sulfide complex (815mL , 8.15mol, 10M in dimethyl sulfide), after the addition, the temperature was raised to room temperature and reacted for 4h to generate a white solid, then the reaction solution was cooled to -40°C, and 3,4-dihydropyran (686g, 8.15mol), after the addition was completed, after rising to room temperature, react for 12h, add anhydrous acetaldehyde (3690g, 81.5mol), react at room temperature for 6h, remove excess anhydrous acetaldehyde under reduced pressure, and then add KHF 2 The saturated solution (1909g, 24.45mol) was reacted at room temperature for 6h, and the solvent was evaporated by a rotary evaporator under reduced pressure, then dried, and the product was extracted with acetone, concentrated under reduced pressure, and ether was added to obtain 1048g of a white sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com