Synthesis method of (2-fluoro-6-(trifluoromethyl) pyridine-3-yl) methanol
A technology of trifluoromethyl and synthesis method is applied in the synthesis field of (2-fluoro-6-(trifluoromethyl)pyridin-3-yl)methanol and achieves the effects of reasonable design, easy control and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] A method for synthesizing (2-fluoro-6-(trifluoromethyl)pyridin-3-yl)methanol, comprising the following steps:
[0019] (1) The mass ratio of compound A and diisopropylamine is 5:3~5, the solid-liquid g / mL ratio of compound A and tetrahydrofuran is 1:14, and the volume ratio of tetrahydrofuran and n-butyllithium in hexane is 5:1, take raw materials, then put diisopropylamine and tetrahydrofuran into the reactor, stir, protect with nitrogen, cool down to -80~-75℃, add n-butyllithium hexane solution, stir for 1~1.5h , then add Compound A, continue to stir for 2-3 hours, pass in carbon dioxide, heat up to 30-35°C, and react for 1-2 hours to obtain Compound B;
[0020] (2) According to the solid-liquid g / mL ratio of compound B and tetrahydrofuran as 1:20, and the volume ratio of borane dimethyl sulfide to tetrahydrofuran as 1:13-15, take raw materials, and then mix compound B and tetrahydrofuran evenly , protected by nitrogen, lower the temperature at -2~0°C, add borane dim...
Embodiment 1
[0022]
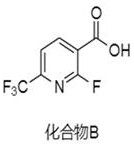
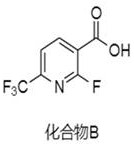
[0023] Preparation of Compound B:
[0024] Put 5g of diisopropylamine and 70mL of tetrahydrofuran into the reactor, stir evenly, under nitrogen protection, cool down to -80°C, add 14mL of n-butyllithium hexane solution (2.5M), stir for 1h, then add 5g of compound A, Continue to stir for 2 hours, replace with carbon dioxide, raise the temperature to 30°C, react for 2 hours, and detect by TLC. After the reaction of the raw materials is complete, add water (50mL) dropwise to quench the reaction, add 5g of potassium carbonate to the reaction solution, and extract impurities with ethyl acetate (50mL *2), the aqueous phase was adjusted to pH 2 with 4M hydrochloric acid, extracted with ethyl acetate (50mL*2), and the organic phase was concentrated to obtain 4.8g of a colorless and transparent oily substance, namely Compound B, with a yield of 75.8% and a purity of 96.8%.
[0025]
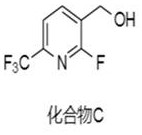
[0026] Preparation of Compound C, i.e. (2-fluoro-6-(trifluoromethyl)pyridin-3-yl)methan...
Embodiment 2
[0031]
[0032] Preparation of compound B:
[0033] Put 4g of diisopropylamine and 70mL of tetrahydrofuran into the reactor, stir evenly, under nitrogen protection, cool down to -75°C, add 14mL of n-butyllithium hexane solution (2.5M), stir for 1.5h, then add 5g of compound A , continue to stir for 3 hours, pass in carbon dioxide, heat up to 35 ° C, react for 2 hours, TLC detection, the reaction of the raw materials is complete, drop water (50 mL) to quench the reaction, add 5 g of potassium carbonate to the reaction solution, and extract impurities with ethyl acetate (50 mL* 2), the aqueous phase was adjusted to pH 2 with 4M hydrochloric acid, extracted with ethyl acetate (50mL*2), and the organic phase was concentrated to obtain 5g of a colorless and transparent oil, namely compound B, with a yield of 78.9% and a purity of 98.2% .
[0034]
[0035] Preparation of compound C, i.e. (2-fluoro-6-(trifluoromethyl)pyridin-3-yl)methanol:
[0036] Mix 3g of compound B and 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com