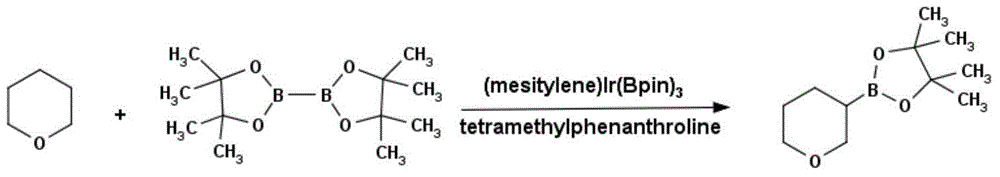
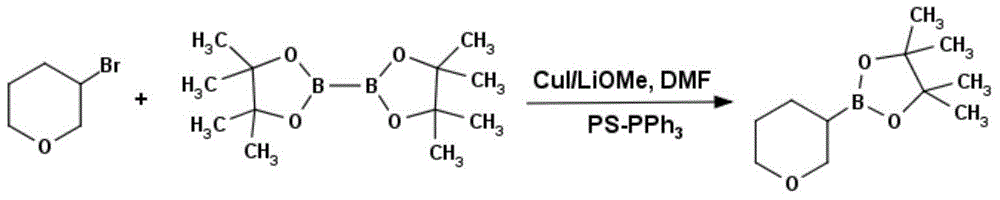
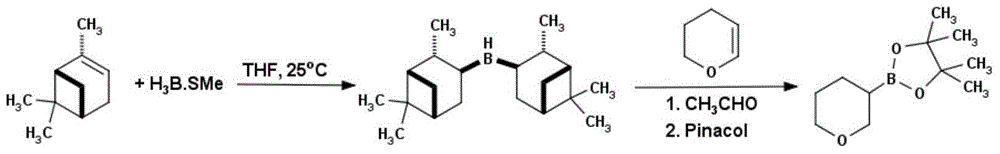
Method for preparing tetrahydropyrane-3-boronic acid pinacol ester
A technology of tetrahydropyran and dihydropyran, which is applied in the field of organic chemical synthesis, can solve the problems of harsh reaction conditions, affecting the reaction yield, and difficulty in obtaining it, and achieves easy industrial production, good process stability, and simple reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 1L three-necked reaction flask, add D-α-pinene (50g, 0.37mol) and 200mL of anhydrous tetrahydrofuran. 0.17mol, 10M in dimethyl sulfide), after the addition, the temperature was raised to room temperature, reacted for 4h, and a white solid was generated, then the reaction solution was cooled to -40°C, and 3,4-dihydropyran (14.3g, 0.17mol), after the addition was completed, after rising to room temperature, reacted for 12h, added anhydrous acetaldehyde (75g, 1.7mol), reacted at room temperature for 6h, removed excess anhydrous acetaldehyde under reduced pressure, and added pinacol (20.1g , 0.17mol), reacted at room temperature for 12h, evaporated the solvent under reduced pressure with a rotary evaporator, separated and purified by silica gel column chromatography, and eluted the product with ethyl acetate:petroleum ether=2:8 to obtain 22.7g of a colorless liquid, namely It is tetrahydropyran-3-boronic acid pinacol ester with a yield of 63%. 1HNMR (CD 3 Cl): 3.89pp...
Embodiment 2
[0029] In a 10L three-necked reaction flask, add D-α-pinene (2331.15g, 17.11mol) and 2.5L anhydrous tetrahydrofuran, under the protection of nitrogen, cool to 0 ° C, slowly dropwise add borane dimethyl sulfide complex ( 815mL, 8.15mol, 10M in dimethylsulfide), the addition was completed, the temperature was raised to room temperature, reacted for 4h, a white solid was generated, then the reaction solution was cooled to -40°C, and 3,4-dihydropyran (686g , 8.15mol), after the addition was completed, after rising to room temperature, reacted for 12h, added anhydrous acetaldehyde (3690g, 81.5mol), reacted at room temperature for 6h, removed excess anhydrous acetaldehyde under reduced pressure, and then pinacol (963g , 8.15mol), reacted at room temperature for 12h, evaporated the solvent under reduced pressure with a rotary evaporator, separated and purified by silica gel column chromatography, and eluted the product with ethyl acetate:petroleum ether=2:8 to obtain 881g of a colorle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com