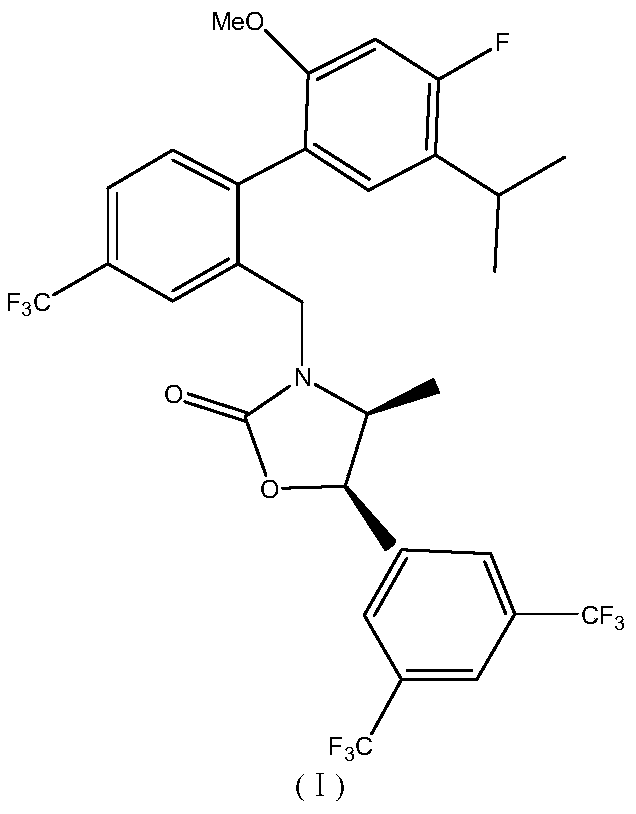
A kind of synthetic method of anseltrapib chiral intermediate
A synthetic method, the technology of anseltrapib, which is applied in the field of medicine, achieves the effects of mild reaction conditions, improved yield and purity, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
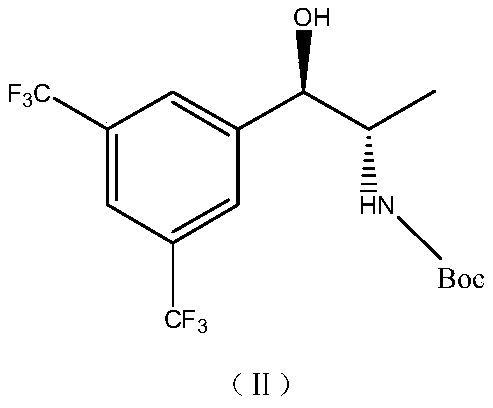
[0033] Preparation of (1R,2S)-1-(3,5-bis(trifluoromethyl)phenyl)-2-Boc-amino-propanol
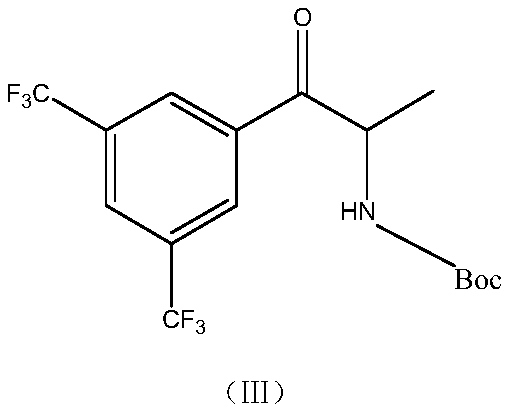
[0034] Under the protection of argon, add 300ml of petroleum ether to the reaction bottle, cool down to -5~0℃, add 3ml (0.01mol) of (R)-2-methyl-CBS-oxazoborane, borane dimethyl sulfide Complex 15ml (0.25mol), stirring for 30min; control 5 ~ 10 ℃ dropwise containing the petroleum ether solution containing 65g (0.162mol) of the compound of formula (Ⅲ); after dropping, stir and react for 1h at 5 ~ 10 ℃, the reaction is complete; cool down To -5 ~ 0 ℃, add methanol dropwise, stir for 30min, add 1mol / L sulfuric acid, stir for 30min. Stand to separate and collect the organic phase; the organic phase is washed once with 1mol / L sulfuric acid, 5% sodium bicarbonate solution and purified water respectively, the obtained organic phase is dried by adding anhydrous sodium sulfate, filtered, and the filtrate is concentrated to dryness under reduced pressure 62.3 g of the crude product was obtained, and...
Embodiment 2
[0036] Preparation of (1R,2S)-1-(3,5-bis(trifluoromethyl)phenyl)-2-Boc-amino-propanol
[0037] Under the protection of argon, add 300ml of acetone to the reaction bottle, cool down to -5~0℃, add 6ml (0.02mol) of (R)-2-methyl-CBS-oxazoborane, borane dimethyl sulfide complex Compound 30ml (0.5mol), stirring for 30min; control 5 ~ 10 ℃ dropwise acetone solution containing 65g (0.162mol) of compound of formula (Ⅲ); after dropping, stir and react for 1h at 5 ~ 10 ℃, the reaction is complete; cool down to - 5~0℃, add ethanol dropwise, stir for 30min, add 1mol / L hydrochloric acid, stir for 30min. Stand for stratification, collect the organic phase; wash the organic phase with 1mol / L hydrochloric acid, 5% sodium bicarbonate solution and purified water successively, add anhydrous sodium sulfate to the obtained organic phase, dry, filter, and concentrate the filtrate to dryness under reduced pressure 63.2 g of the crude product was obtained, and the (R, S) / (R, R) configuration ratio de...
Embodiment 3
[0039] Preparation of (1R,2S)-1-(3,5-bis(trifluoromethyl)phenyl)-2-Boc-amino-propanol
[0040] Under the protection of argon, add 300ml of chloroform into the reaction flask, cool down to -5~0℃, add 10ml (0.034mol) of (R)-2-methyl-CBS-oxazoborane, borane dimethyl sulfide Ether complex 30ml (0.5mol), stirred for 30min; controlled 5 ~ 10 ℃, dropwise added chloroform solution containing 65g (0.162mol) of the compound of formula (Ⅲ); after dropping, stirred and reacted for 1h at 5 ~ 10 ℃, the reaction was complete ; Cool down to -5~0°C, add methanol dropwise, stir for 30 minutes, add 1mol / L sulfuric acid, and stir for 30 minutes. Stand to separate and collect the organic phase; the organic phase was washed once with 1mol / L sulfuric acid, 5% sodium bicarbonate solution and purified water respectively, the obtained organic phase was dried by adding anhydrous magnesium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure 63.5 g of the crude product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com