Stabilizer-containing borane reagent combination solution, and preparation method and use thereof
A technology of stabilizer and borane dimethyl sulfide complex, applied in the field of combined solutions, can solve problems such as non-obviousness, and achieve the effects of increasing stability, reducing waste and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 borane dimethyl sulfide complex (BDMS)
[0036] Purge the glass reaction flask with nitrogen and simultaneously charge 660 grams of dimethyl sulfide, lower the temperature in the reaction flask to 0°C, and bubble 138 grams of diborane into the reaction flask after 1 hour. During the process, the temperature was controlled at 0-5° C., and the stirring reaction was continued for 15 minutes after the introduction of diborane was completed. After detection, the density of the borane dimethyl sulfide complex obtained by the reaction is 0.80 g / mL, and the concentration of borane (ie, the borane dimethyl sulfide complex) is 10.08 mol / liter (M).
Embodiment 2
[0037] The preparation of embodiment 2 borane tetrahydrofuran complexes (BTHF)
[0038] The glass reaction flask was purged with nitrogen and 353.8 grams of tetrahydrofuran was charged at the same time, the temperature in the reaction flask was lowered to 0° C., and diborane (11.8 grams) was bubbled into the reaction flask for 0.5 hours. During the bubbling process, , control the temperature at 0-5° C., and continue stirring for 15 minutes after the diborane is passed through. After testing, the density of the solution after the reaction was 0.87 g / mL, and the concentration of borane (ie borane tetrahydrofuran complex) was 2 mol / L.
[0039] Add sodium borohydride to the borane tetrahydrofuran complex solution prepared above, so that the final concentration of sodium borohydride in the solution is 0.005M, that is, the molar ratio of sodium borohydride to borane tetrahydrofuran complex is 1:400 . After the addition of sodium borohydride was complete, the solution was stirred f...
Embodiment 3
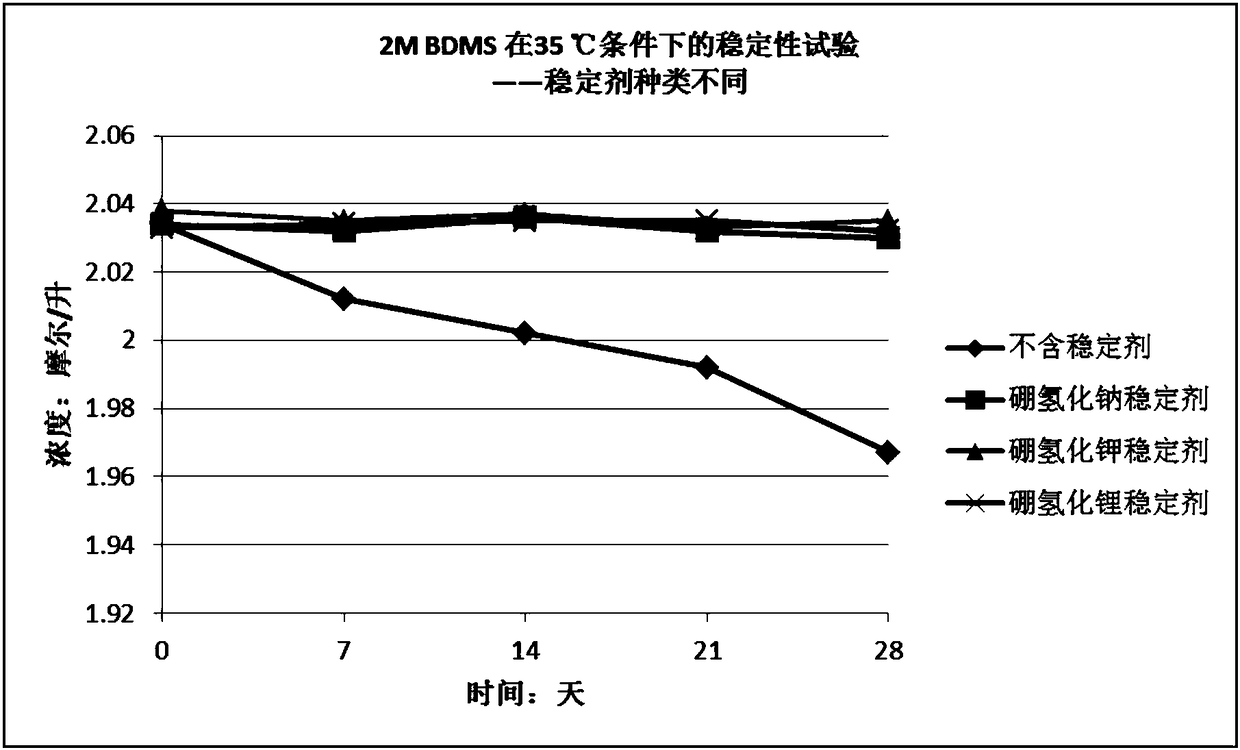
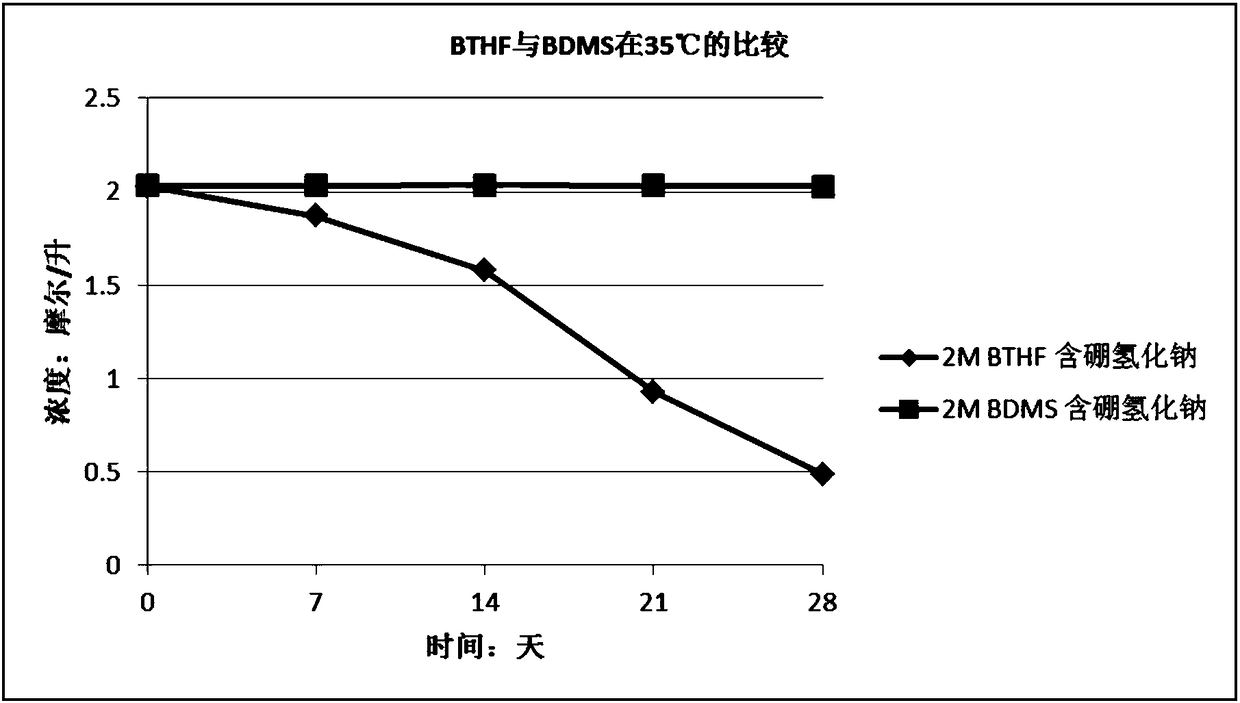
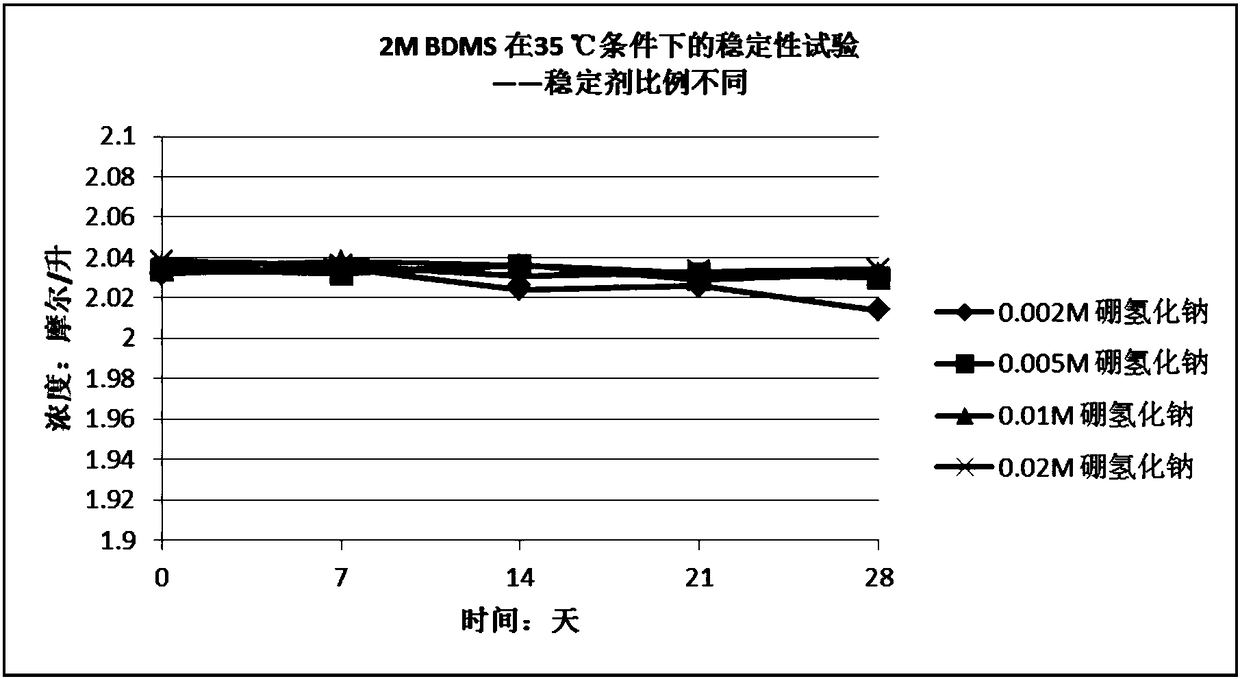
[0040] Embodiment 3 Stabilizer type (sodium borohydride, potassium borohydride and lithium borohydride) is on the influence of the stability of the tetrahydrofuran solution of borane dimethyl sulfide complex and under the same conditions, the tetrahydrofuran of borane tetrahydrofuran complex Comparison of Stability of Solution and Tetrahydrofuran Solution of Borane Dimethyl Sulfide Complex
[0041] Using tetrahydrofuran as a solvent, dilute the borane dimethyl sulfide complex obtained in Example 1 to about 2 mol / liter, then divide the diluted solution into four equal parts, and add Sodium borohydride, potassium borohydride, lithium borohydride, so that the final concentration of the three stabilizers in the solution is 0.005M, that is, the molar ratio of borane dimethyl sulfide complex to sodium borohydride is 400:1. After adding sodium borohydride and lithium borohydride, the solution was stirred for 1 hour to dissolve the stabilizer to obtain solutions B and C; after adding ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com