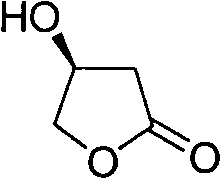
Synthesis method of S-beta-hydroxy-gamma-butyrolactone
A synthesis method and technology of methyl hydroxybutyrate are applied in the field of synthesizing -β-hydroxy-γ-butyrolactone, which can solve the problems of high price, flammability, explosion, and difficulty in storage, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of synthetic method of S-β-hydroxyl-γ-butyrolactone, comprises the steps:
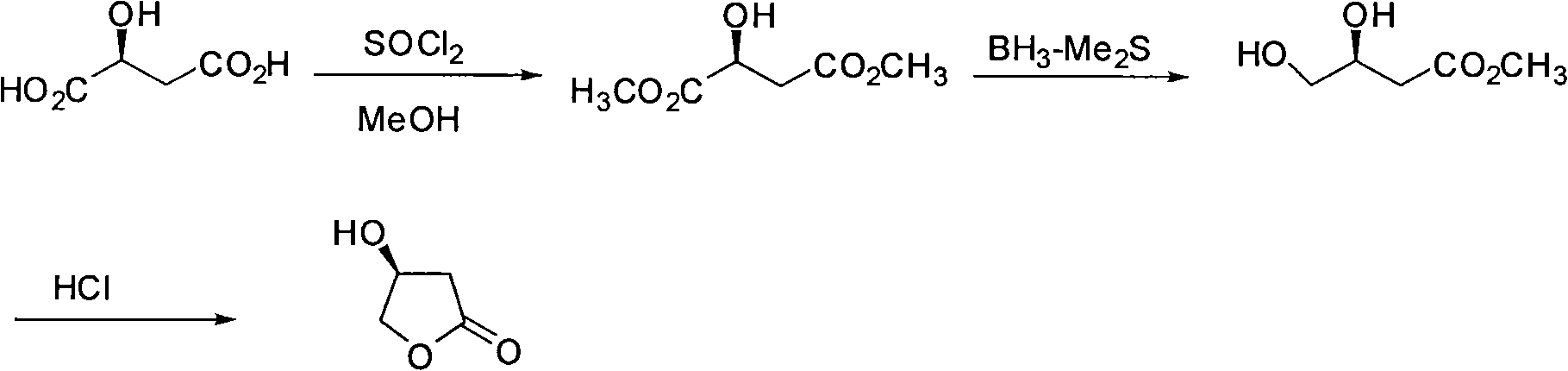
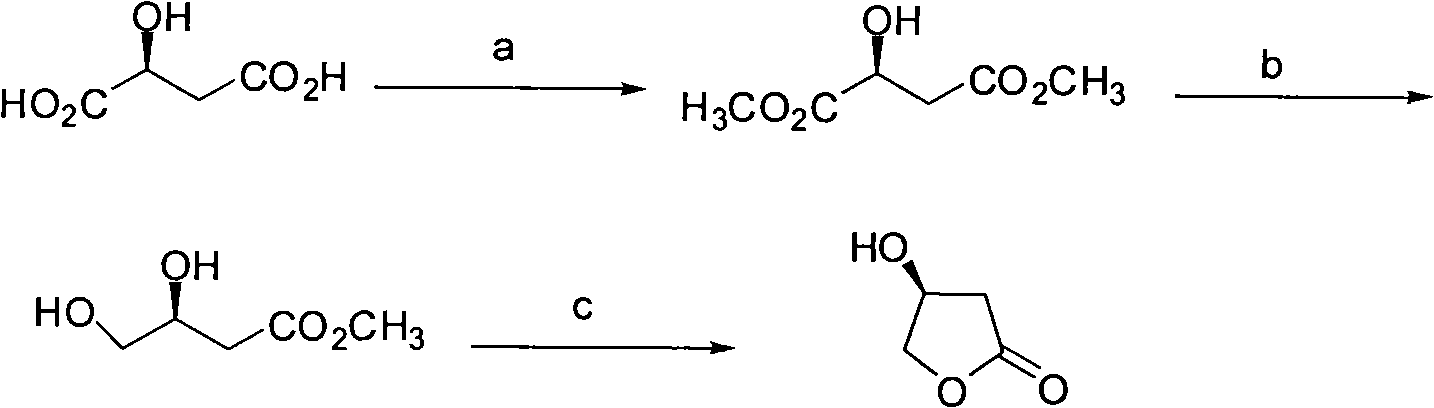
[0048] (1), the synthesis of L-dimethyl malate
[0049] Acetyl chloride (10ml) was slowly added to methanol (300ml) at 0°C. After dropping, L-malic acid (26.8 g, 0.2 mol) was added, slowly raised to room temperature, and stirred for 12 h. Methanol was concentrated, the residue was dissolved in 250ml of ethyl acetate, the organic phase was washed with saturated sodium bicarbonate, water and saturated sodium chloride solution, dried and concentrated to obtain an intermediate. Dissolve the intermediate in 2.5ml THF, add tetrabutylammonium fluoride (1.0M, 0.6ml, 0.6mmol) dropwise at 0°C, stir the mixture at 0°C for 3 hours, dilute with 15ml ether, and chlorinate the organic phase with water and saturated Washed with sodium solution, dried and concentrated to obtain 27 g of colorless oily liquid with a yield of 92%.
[0050] [α] D 25 = -7.50 (c 3.45, MeOH);
[0051] 1 H NMR (CDCl 3 , 3...
Embodiment 2
[0064] A kind of synthetic method of S-β-hydroxyl-γ-butyrolactone, comprises the steps:
[0065] (1), the synthesis of L-dimethyl malate
[0066] Acetyl chloride (5ml) was slowly added to methanol (300ml) at 0°C. After dropping, L-malic acid (26.8 g, 0.2 mol) was added, slowly raised to room temperature, and stirred for 12 h. Methanol was concentrated, the residue was dissolved in 250ml of ethyl acetate, the organic phase was washed with saturated sodium bicarbonate, water and saturated sodium chloride solution, dried and concentrated to obtain an intermediate. Dissolve the intermediate in 2ml of THF, add tetrabutylammonium fluoride (1.0M, 0.5ml, 0.5mmol) dropwise at 0°C, stir the mixture at 0°C for 3 hours, dilute with 15ml of ether, and the organic phase with water and saturated sodium chloride The solution was washed, dried and concentrated to obtain 26.4 g of a colorless oily liquid with a yield of 90%.
[0067] [α] D 25 = -7.50 (c 3.45, MeOH);
[0068] 1 H NMR (CDC...
Embodiment 3
[0081] A kind of synthetic method of S-β-hydroxyl-γ-butyrolactone, comprises the steps:
[0082] (1), the synthesis of L-dimethyl malate
[0083] Acetyl chloride (10ml) was slowly added to methanol (500ml) at 0°C. After dropping, L-malic acid (26.8 g, 0.2 mol) was added, slowly raised to room temperature, and stirred for 12 h. Methanol was concentrated, the residue was dissolved in 250ml of ethyl acetate, the organic phase was washed with saturated sodium bicarbonate, water and saturated sodium chloride solution, dried and concentrated to obtain an intermediate. Dissolve the intermediate in 5ml of THF, add tetrabutylammonium fluoride (1.0M, 1.0ml, 1.0mmol) dropwise at 0°C, stir the mixture at 0°C for 3 hours, dilute with 15ml of ether, and the organic phase with water and saturated sodium chloride The solution was washed, dried and concentrated to obtain 26.7 g of a colorless oily liquid with a yield of 91%.
[0084] [α] D 25 = -7.50 (c 3.45, MeOH);
[0085] 1 H NMR (CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com