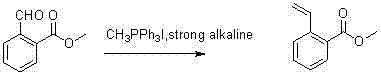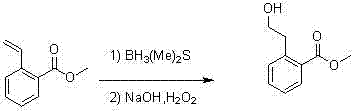Synthesis method for 2-(2-bromoethyl)benzoic acid methyl ester
A technology of vinyl methyl benzoate and formaldehyde methyl benzoate, which is applied in the field of synthesis of 2-methyl benzoate and can solve problems such as unfavorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0012] Methyltriphenylphosphine hydriodide (36.9 g, 91.5 mmol) was suspended in THF (500 mL), -50 o 1.6 mol per liter of n-butyllithium (57.2 ml, 91.5 mmol) was added dropwise at 0°C. After the addition, the reaction mixture was slowly warmed to room temperature and stirred for an hour, then continued to cool to -70 o C. A solution of methyl 2-formaldehyde benzoate (10 g, 61 mmol) in tetrahydrofuran (150 ml) was added dropwise to the above solution. After the addition, the temperature was slowly raised to room temperature and stirred overnight. After the reaction solution was concentrated, 500 ml of water was added. , extracted twice with dichloromethane, the organic phase was dried and concentrated to obtain an oil, and a colorless oily product, methyl 2-vinylbenzoate (7 g, yield 70%) was obtained after silica gel column chromatography.
[0013] Methyl 2-vinylbenzoate (7 g, 43.2 mmol) obtained in the previous step was dissolved in tetrahydrofuran (45 ml), cooled to 0°C, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com