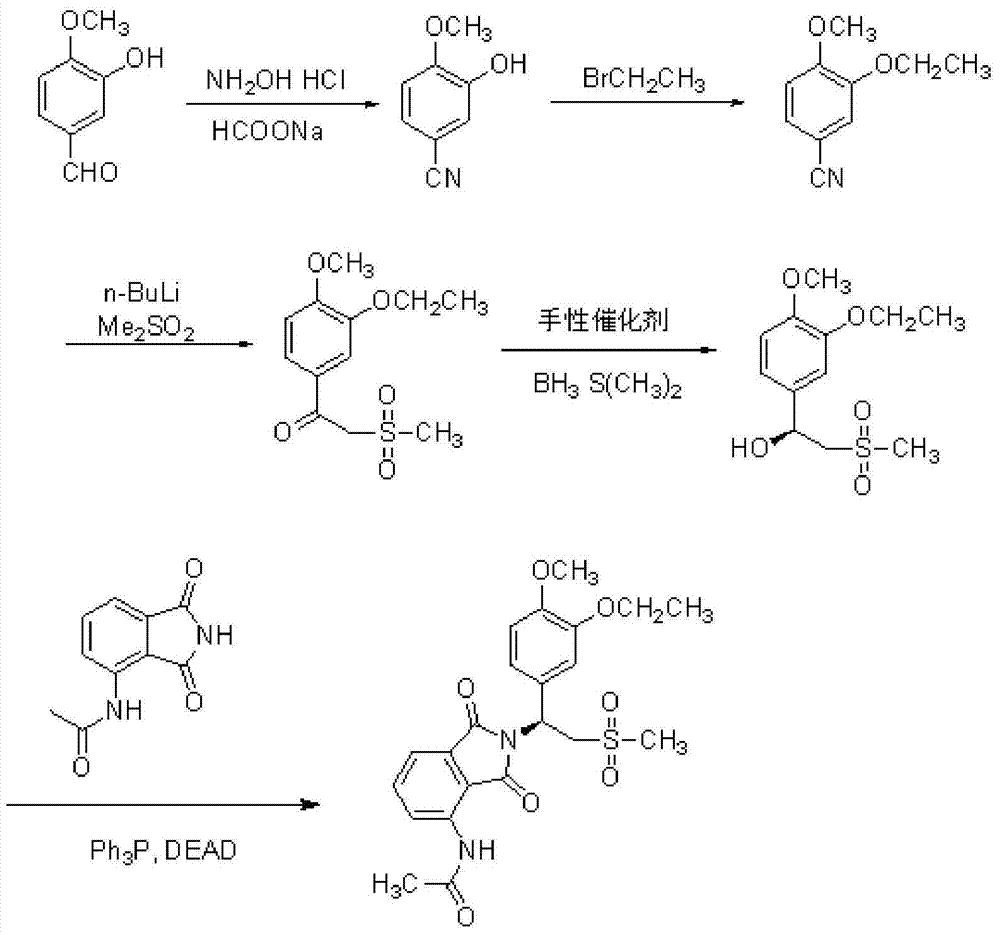
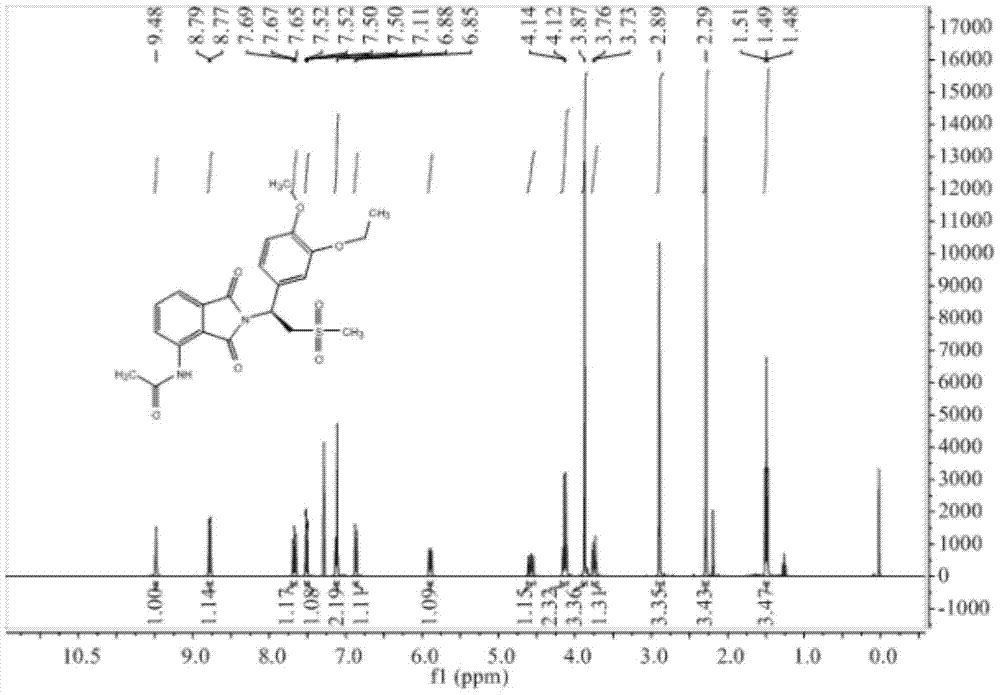
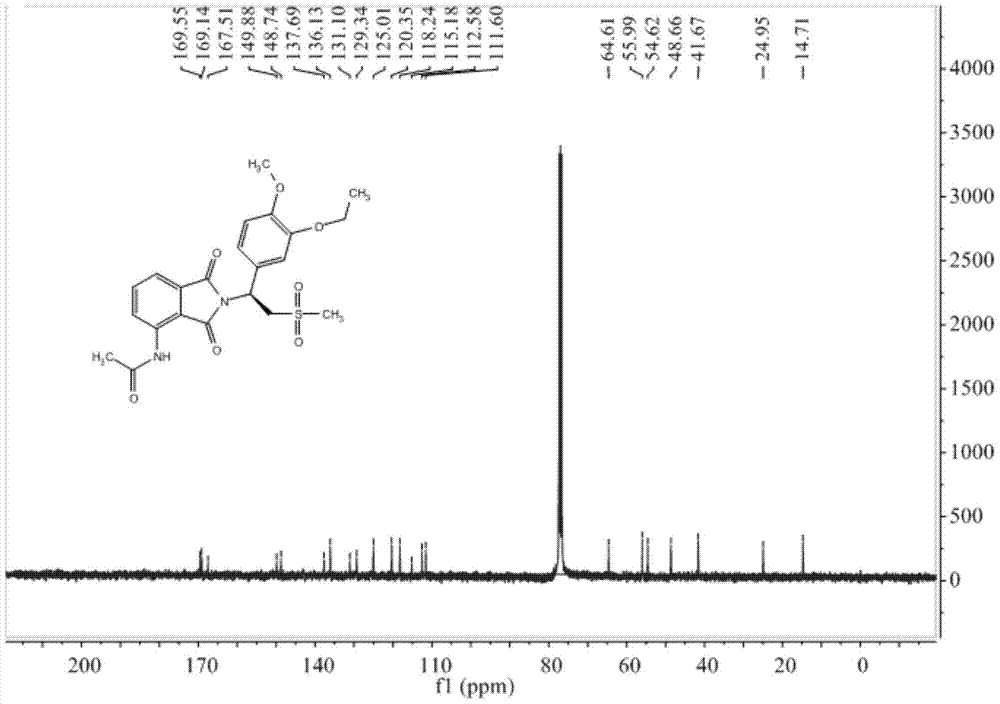
A kind of preparation method of Apremilast
A hydroxyl and molar ratio technology, applied in the field of psoriatic arthritis drug preparation, can solve the problems of expensive catalysts, unfavorable industrial production, and high production costs, and achieve the effects of low cost, easy industrial production, and short reaction routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] In a 100mL three-neck flask, add 10.04g (65.99mmol) of 3-hydroxy-4-methoxybenzaldehyde, 45mL of formic acid, and 13.75g (132.16mmol) of sodium formate in sequence, heat and stir to 85°C, and all the reactants have been dissolved in formic acid middle. At 85° C., 6.12 g (88.07 mmol) of hydroxylamine hydrochloride was added, and the reaction was monitored by TLC, and the reaction ended after 5 hours. Stop heating, cool to room temperature, add the reaction solution into 200mL saturated brine, stir for 30min; suction filter, wash the solid with water until neutral, dry to obtain 9.04g of white solid, yield 92%, mp: 129~132°C. IR (cm -1 ,KBr): 3320, 2930, 2280, 1611, 1578, 1510cm -1 ; 1 H NMR (400MHz, CDCl 3 ): δ3.98(s,3H),5.78(s,1H),6.92(d,J=8.3Hz,1H),7.26–7.17(m,2H); 13 C NMR (101MHz, CDCl 3 )δ56.16, 104.64, 110.77, 117.64, 119.01, 125.63, 145.95, 150.24; EI-MS: 149[M + ].
Embodiment 2
[0058] In a 100mL single-necked flask, add 10g (67.11mmol) of 3-hydroxy-4-methoxybenzonitrile, 25mL (335.2mmol) of ethyl bromide, 10.25g of potassium carbonate, and 50mL of dimethylformamide in sequence, and heat and stir until 100°C. TLC monitored the reaction, reacted for 8h, and stopped heating. Naturally cooled to room temperature, added 100 mL of water, extracted with ethyl acetate, dried the organic phase with anhydrous sodium sulfate, and spin-dried the solvent ethyl acetate to obtain 11.09 g of a white solid with a yield of 94%, mp: 68-70°C. 1 H NMR (400MHz, CDCl 3 )δ: 1.49(t, J=6.9Hz, 3H), 3.92(s, 3H), 4.10(dd, J=13.6, 6.7Hz, 2H), 6.91(d, J=8.3Hz, 1H), 7.08( s,1H),7.27(d,J=6.9Hz,1H); 13 C NMR (101MHz, CDCl 3 ): δ14.48, 56.04, 64.75, 103.94, 111.51, 115.40, 119.26, 126.31, 148.43, 153.06; EI-MS: 177[M + ].
Embodiment 3
[0060] Add 2.6g (28.3mmol) of dimethyl sulfone and 10mL of tetrahydrofuran to the reactor, cool down to 0-10°C under the protection of nitrogen, add 20mL of 1.6M n-butyllithium n-hexane solution to the reactor, and control the temperature at Stir at 0-10°C for 3h, then dissolve 4.0g (22.60mmol) of the obtained 3-ethoxy-4-methoxybenzonitrile in 10mL of tetrahydrofuran and add dropwise to the above reaction solution at 0-10°C. After the addition, the temperature of the reaction system was raised to room temperature, stirred for 6 hours, until the reaction was complete, then hydrochloric acid solution was added dropwise to quench the reaction, after stirring for 30 minutes, the solvent was spin-dried, and water was added for suction filtration to obtain 4.96 g of white solid 1-(3- Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanone, yield 81%, mp: 118-120°C. 1 H NMR (400MHz, CDCl 3 )δ1.52(t, J=7.0Hz, 3H), 3.16(s, 3H), 3.99(s, 3H), 4.19(q, J=7.0Hz, 2H), 4.57(s, 2H), 6.98( t, J=13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com