A kind of lorcaserin hydrochloride pellets, its preparation method and its preparation
A technology of lorcaserin hydrochloride and lorcaserin hydrochloride, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with non-active ingredients, etc., can solve problems such as unfavorable health of patients and large fluctuation of drug concentration peaks and valleys.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
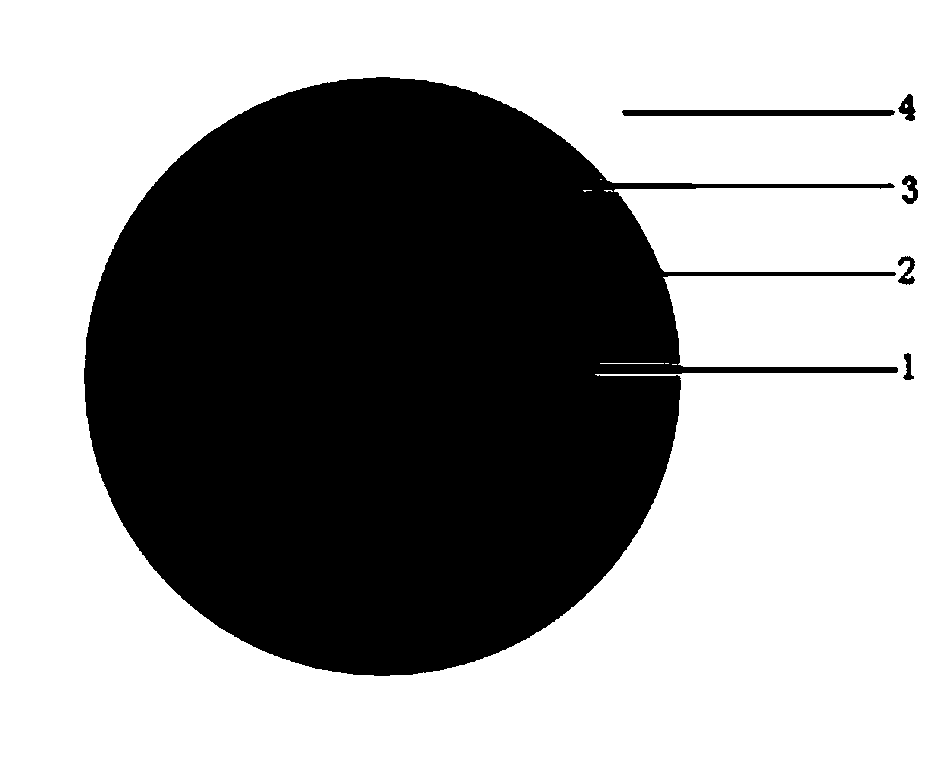
[0060] The present invention also provides a preparation method of lorcaserin hydrochloride pellets, comprising the following steps:
[0061] mixing lorcaserin hydrochloride, lactose monohydrate, highly substituted hydroxypropyl cellulose, and talcum powder with a first solvent to obtain a drug-loaded layer solution, and the first solvent includes water and ethanol;
[0062] Mixing ethyl cellulose, highly substituted hydroxypropyl cellulose, talcum powder, and triethyl citrate with a second solvent to obtain a sustained-release layer solution, the second solvent including ethanol;
[0063] mixing lorcaserin hydrochloride, lactose monohydrate, highly substituted hydroxypropyl cellulose, and talc with a third solvent to obtain an immediate-release layer solution, the third solvent including water and ethanol;
[0064] The drug-loading layer solution, the slow-release layer solution and the quick-release layer solution are coated on the blank pill cores in sequence by a drug-on-s...
Embodiment 1
[0084] The preparation of embodiment 1 lorcaserin hydrochloride
[0085] Add 1.5L of methanol and 150g of isopropanolamine into a 5L reaction vessel, stir evenly, add 307.7g of p-chlorophenylacetic acid, heat to 50°C, keep warm for 6 hours, recover the solvent under reduced pressure, add purified water, and control the temperature at 20°C Crystallize, filter, and dry the filter cake to obtain 368.5 g of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide with a purity of 96.8%.
[0086]After obtaining 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide, 1.5L toluene and 342g2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide Mix, stir to dissolve, cool down to 0°C in an ice-water bath, slowly add 150 g of thionyl chloride (halogenation reagent) dropwise, after the dropwise addition, add 15 mL of triethylamine, control the temperature at 15°C, react for 2 hours, recover the solvent, and then add 1.5L 2-Methyltetrahydrofuran, 600mL 1mol / L sodium hydroxide solution, separate the liquid, dry...
Embodiment 2
[0090] The preparation of embodiment 2 lorcaserin hydrochloride pellets
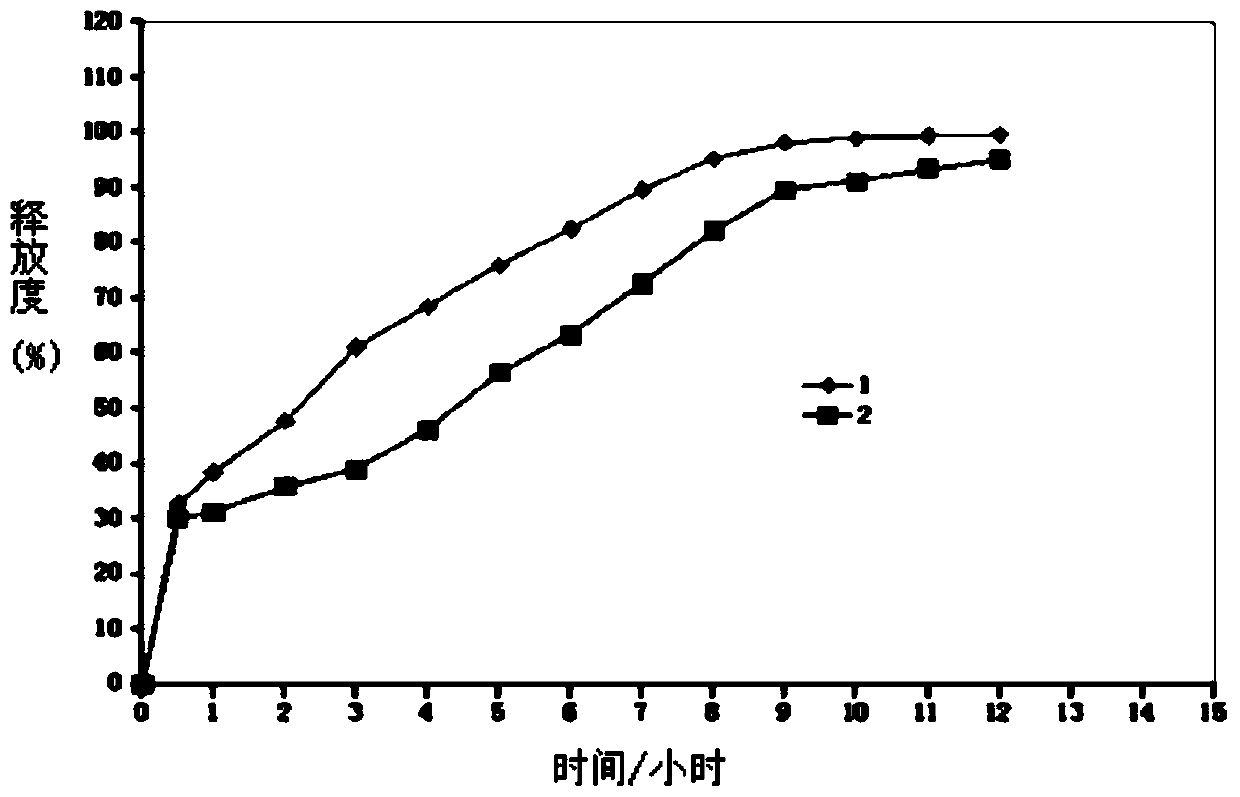
[0091] Take 15.8g of high-substituted hydroxypropyl cellulose and add it to 750g of purified water at 50°C, stir and swell until it becomes clear, and cool to the greenhouse for use; add 63g of lorcaserin hydrochloride and 63g of lactose monohydrate, stir well, and obtain a suspension liquid. Pass the above suspension through an 80-mesh sieve, rinse it with 375g of 95% ethanol, add 375g of 95% ethanol, and stir evenly to obtain a drug-loaded layer solution. Fill 750g of sucrose-starch microspheres into the fluidized bed granulator coating machine, the fan frequency is 30Hz, the air inlet temperature is 60°C, the material temperature is 35°C, the atomizing pressure is 0.18MPa, and the spray pump speed is 6 rpm. Drug operation (coating) until the drug-loaded layer solution is sprayed out. Set the material temperature at 40°C, fluidize and dry the pellets coated with the drug-loaded layer solution for 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com