Lorcaserin hydrochloride sustained-release capsules and preparation method thereof
A technology of lorcaserin hydrochloride and sustained-release capsules, which is applied in the field of lorcaserin hydrochloride sustained-release capsules and its preparation, can solve the problems such as no research and reports on lorcaserin hydrochloride sustained-release capsules, and achieve suitable For industrialized production, simple operation and reliable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of lorcaserin hydrochloride sustained-release pellets
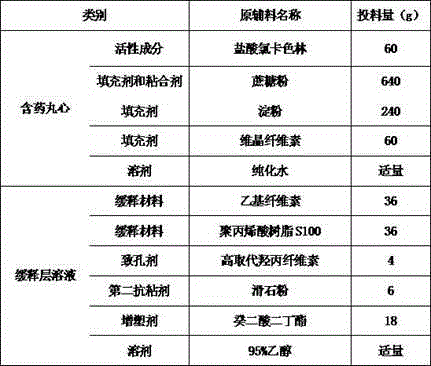
[0028] Take an appropriate amount of pure water and pour it into the batching pot, add 64g of sucrose while stirring and heating to make 50-60% syrup. When the sucrose is all dissolved, continue to heat until it boils. Pass through a 200-mesh sieve in a pre-cleaned stainless steel bucket, let it cool, and set aside.
[0029] Put 60g of lorcaserin hydrochloride (or its hemihydrate), 576g of sucrose, 240g of starch, and 60g of microcrystalline cellulose into a mill for grinding, pass the pulverized material through a 80-100 mesh sieve, and set aside.
[0030] Put the pulverized and sieved material into a wet granulator and mix for 5 minutes, add the prepared syrup above to make a soft material, put the soft material into the extrusion equipment with a screen mesh of 14-16 mesh, and extrude the strip material. The strip material is put into the spheronizing equipment to prepare the pill core co...
Embodiment 2
[0038] Example 2 Preparation of lorcaserin hydrochloride sustained-release pellets
[0039]Take an appropriate amount of pure water and pour it into the batching pot, add 60g of sucrose while stirring and heating to make a 50-60% syrup. When the sucrose is all dissolved, continue to heat until it boils. Pass through a 200-mesh sieve in a pre-cleaned stainless steel bucket, let it cool, and set aside.
[0040] Put 60g of lorcaserin hydrochloride (or its hemihydrate), 540g of sucrose, 200g of starch, and 140g of microcrystalline cellulose into a mill for grinding, pass the pulverized material through an 80-100 mesh sieve, and set aside.
[0041] Put the pulverized and sieved material into a wet granulator and mix for 5 minutes, add the prepared syrup above to make a soft material, put the soft material into the extrusion equipment with a screen mesh of 14-16 mesh, and extrude the strip material. The strip material is put into the spheronizing equipment to prepare the pill core...
Embodiment 3
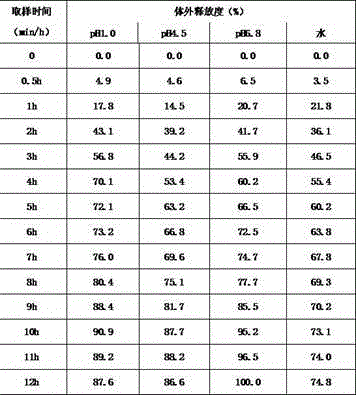
[0044] Example 3 Determination of In Vitro Release of Lorcaserin Hydrochloride Sustained-release Capsules
[0045] Get 6 lorcaserin hydrochloride (or its hemihydrate) sustained-release capsules prepared in Example 1, and operate according to the dissolution method (the second method of appendix X C of "Chinese Pharmacopoeia" 2010 edition): measure 900mL of solutions with different pH are used as the dissolution medium, the rotation speed is 100 revolutions per minute, according to the operation, after 30min, 1h, 2h, 3h, 4h, 5h, 6h, 7h, 8h, 9h, 10h, 11h, 12h, each solution is taken Add 10mL of the dissolution medium at the same temperature and volume at the same time, filter, accurately measure the appropriate amount of the filtrate, and measure it according to HPLC (Appendix V D of Part Two of the Chinese Pharmacopoeia, 2010 Edition). Detection wavelength: 222nm, column temperature 35°C, flow rate 1.0ml / min; another precisely weighed lorcaserin hydrochloride reference substanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com