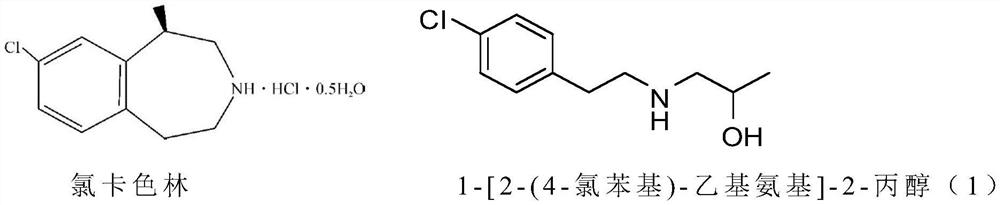
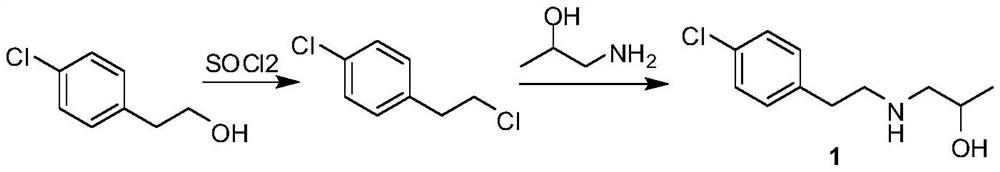
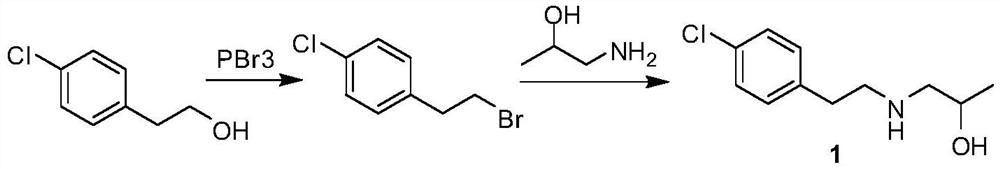
Preparation method of lorcaserin intermediate
A technology for lorcaserin and intermediates, which is applied in the field of preparation of 1-[2--ethylamino]-2-propanol, an intermediate of lorcaserin for weight loss, can solve waste, environmental protection pressure, increase Production costs and other issues, to achieve the effect of simple operation, good safety, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add p-chlorophenylethanol (156.6 g, 1 mol) and 780 ml of 40% hydrobromic acid into the reaction flask, stir and heat up to 80° C., and react for 72 h. The reaction solution was cooled to room temperature, allowed to stand for liquid separation, and the organic phase was washed with water until neutral to obtain 215 g of 4-chlorophenylethyl bromide as a pale yellow oil, with a yield of 98%.
Embodiment 2
[0019] Add 4-chlorophenylethyl bromide (215g, 0.98mol), anhydrous potassium carbonate (149g, 1.08mol), potassium iodide (8.1g, 0.049mol) into the reaction flask, stir and heat up to 60°C, and dropwise add iso Propanolamine (77.3 g, 1.03 mol) was reacted at 60° C. for 4 h after dropping. Cool the reaction solution to room temperature, add 200ml of water to it, stir, and filter to obtain off-white solid 1-[2-(4-chlorophenyl)-ethylamino]-2-propanol, which is air-dried at 50°C to obtain 178g, yield 85%.
Embodiment 3
[0021] Add p-chlorophenylethanol (156.6 g, 1 mol) and 1250 ml of 40% hydrobromic acid into the reaction flask, stir and heat up to 90° C., and react for 48 h. The reaction solution was cooled to room temperature, allowed to stand for liquid separation, and the organic phase was washed with water until neutral to obtain 217 g of 4-chlorophenylethyl bromide as a light yellow oil, with a yield of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com