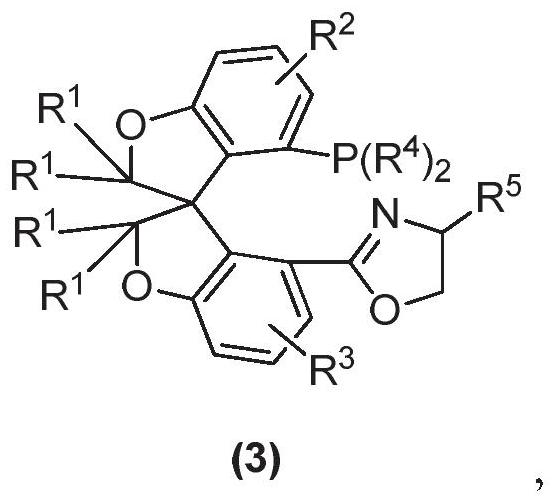
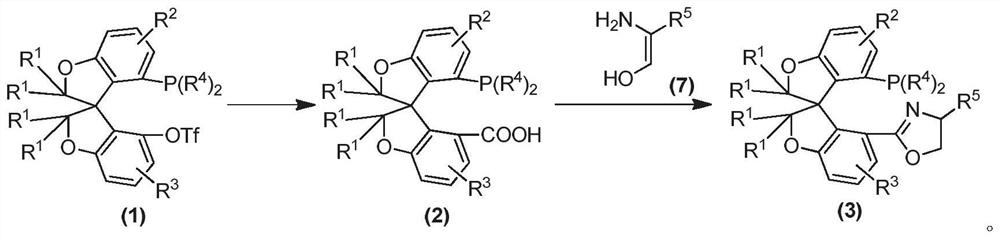
Oxa-spiro phosphine-oxazoline ligand as well as preparation method and application thereof
An oxaspiro and oxazoline technology is applied in the field of oxaspirophosphine-oxazoline ligands and their preparation, and can solve the problems of low total yield, long reaction steps, low product ee value and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 compound (S a )-2b preparation
[0085]
[0086] Under an argon atmosphere, the compounds (S a )-1b (3.90 g, 7.0 mmol), methanol (42 mL), palladium acetate (259 mg, 1.16 mmol), dppp (478 mg, 1.46 mmol), dimethylsulfoxide (60 mL) and triethylamine (11.6 mL). The reaction mixture was reacted in a carbon monoxide atmosphere at 70° C. until the reaction was complete. Cool to room temperature, extract three times with ethyl acetate, and then remove the solvent in the mixture under reduced pressure, and the crude product is directly hydrolyzed in the presence of potassium hydroxide. Finally, the compound (S a )-2b (2.02 g, yield 64%);
[0087] [α] 25 D = -32.00 (c = 0.5, acetone);
[0088] 1 H NMR (400MHz, Chloroform-d): δ7.36-7.17(m, 4H), 7.17-7.00(m, 2H), 6.94(d, J=6.5Hz, 1H), 6.76(t, J=8.5Hz , 4H), 6.54(s, 1H), 4.98(d, J=8.4Hz, 1H), 4.74(d, J=8.3Hz, 2H), 4.62(d, J=8.5Hz, 1H), 1.15(s , 18H), 1.13(s, 18H). 13C NMR (101MHz, Chloroform-d): δ162.2, ...
Embodiment 2
[0090] Embodiment 2 compound (R a )-2a preparation
[0091]
[0092] According to the method described in Example 1, the compound (R a )-1a prepared compound (R a )-2a, the detection data are as follows:
[0093] [α] 25 D =+23.40 (c=0.5, methanol);
[0094] 1 H NMR (400MHz, Chloroform-d): δ7.32-7.22 (m, 4H), 7.22-7.14 (m, 3H), 7.13-7.01 (m, 4H), 6.90-6.79 (m, 2H), 6.67 ( m, 1H), 6.29(dd, J=7.9, 0.8Hz, 1H), 5.73(dd, J=8.0, 0.8Hz, 1H), 5.01(dd, J=9.2, 2.6Hz, 1H), 4.67(d , J=9.3Hz, 1H), 4.62(dd, J=9.2, 1.3Hz, 1H), 4.53(d, J=9.4Hz, 1H). 13 C NMR (101MHz, Chloroform-d): δ161.29, 161.27, 160.25, 160.2, 143.5, 137.1, 137.0, 135.9, 135.7, 135.1, 135.0, 134.1, 133.9, 133.3, 133.1, 132.5, 1394.2, 139.0. 128.7, 128.29, 128.25, 128.2, 128.1, 128.0, 127.8, 127.7, 110.9, 108.8, 83.3, 80.7, 56.1. 31 P NMR (162MHz, Chloroform-d): δ-22.55;
[0095] HRMS(ESI)calcd.for C 28 h 20 o 4 P[M-H] - : 451.1105.Found: 451.1104.
Embodiment 3
[0096] Embodiment 3 compound (S a , Preparation of S)-3d
[0097] Under the protection of argon, add (S a )-2b (1000 mg, 1.48 mmol), (S)-(+)-2-phenylglycinol (637 mg, 4.65 mmol), HOBt (504 mg, 3.29 mmol), DCC (881 mg, 4.27 mmol) and 80 mL THF. The reaction mixture was reacted at 40° C. for 24 hours, and then cooled to room temperature. The reaction mixture was concentrated under reduced pressure to remove the solvent, and separated and purified by column chromatography. Then the obtained product and 0.32 mL of triethylamine, 4-dimethylaminopyridine (5 mg, 0.04 mmol) were added into 65 mL of dichloromethane. Methanesulfonyl chloride (120 μL, 1.55 mmol) was added to the mixture at 0° C., stirred for 30 minutes, and then 1.35 mL of triethylamine was added, warmed to room temperature, and stirred until the reaction was completed. Carry out separation and purification by column chromatography, can obtain product (S a , S)-3d (678mg, yield 59%);
[0098] [α] 25 D =-154.40 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com