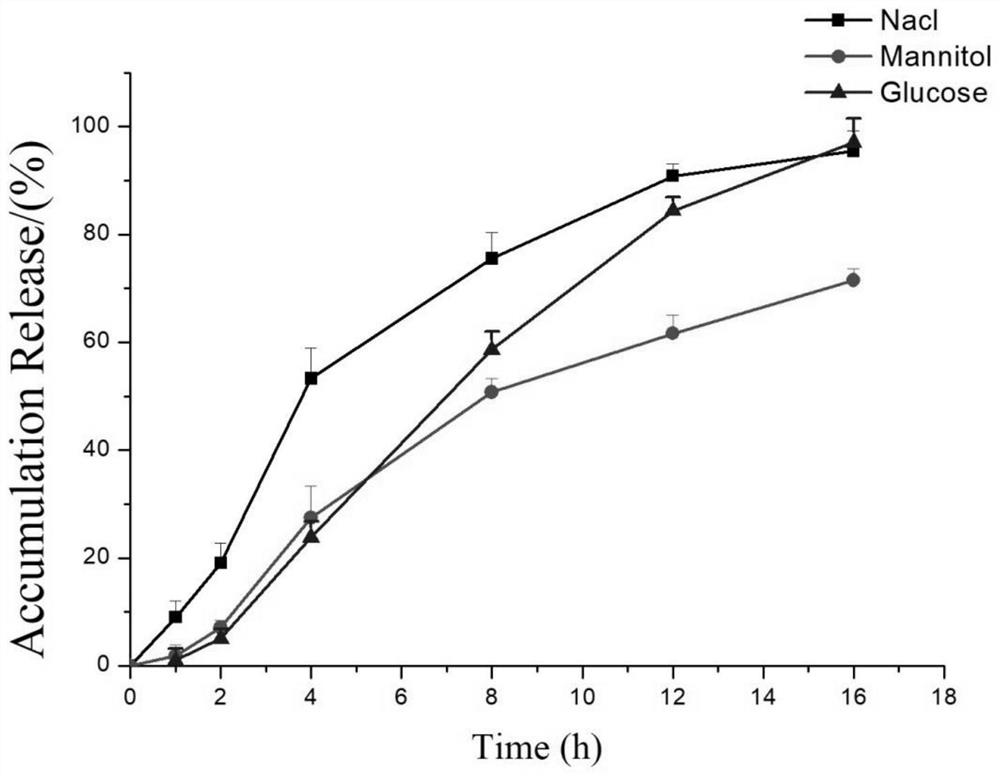
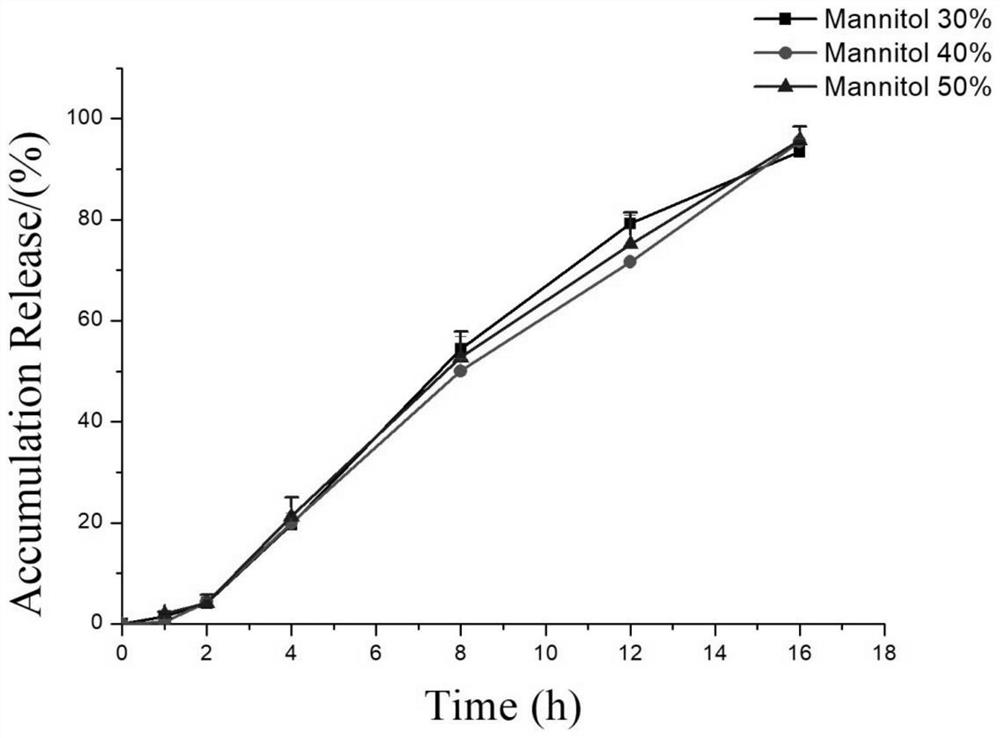
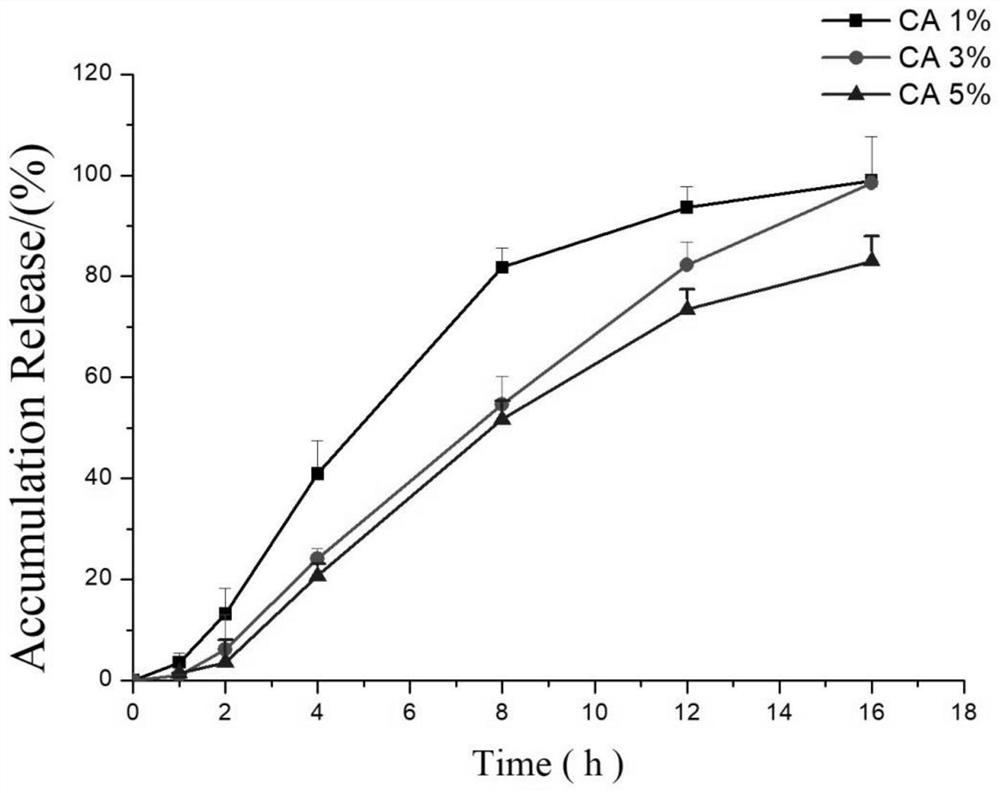
Lorcaserin hydrochloride osmotic pump controlled release preparation and preparation method thereof
A lorcaserin hydrochloride and osmotic pump controlled release technology, which is applied in the field of medicine, can solve problems such as body dependence, damage to the release mechanism of sustained-release tablets, and sudden drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] A 250 mg dose of lorcaserin hydrochloride osmotic pump tablets was prepared.
[0074] Tablet core: pass 20mg of lorcaserin hydrochloride, 100mg of mannitol, 7.5mg of polyvinylpyrrolidone, and 117.5mg of microcrystalline cellulose through an 80-mesh sieve, weigh them and mix them in equal amounts, add 250mg of 70% ethanol solution to make a soft material , granulated with a 20-mesh sieve, dried at 40°C for 6 hours, added with 5g of magnesium stearate and mixed evenly.
[0075] Preparation of coating solution: Weigh 10g of cellulose acetate and dissolve it in 500ml of acetone to prepare a solution of cellulose acetate in acetone, continue to add 0.75g of dibutyl phthalate DBP and 1g of PEG400, and place it on a magnetic stirrer without heating Stir under conditions until the solution is completely dissolved.
[0076] Tablet compression and coating: install the upper and lower punches, adjust the punching pressure to 60N, fill in 250 mg of tablet core granules and press i...
Embodiment 2
[0078] Pass 18mg of lorcaserin hydrochloride, 90mg of mannitol, 7mg of polyvinylpyrrolidone, and 110mg of microcrystalline cellulose through an 80-mesh sieve, weigh them according to the prescription amount, mix them by equal addition method, add 200mg of 70% ethanol solution to make a soft material, and use Granulate with a 20-mesh sieve, dry at 40°C for 6 hours, add 4g of magnesium stearate and mix well.
[0079] Preparation of coating solution: Dissolve 8g of cellulose acetate in 500ml of acetone to prepare a cellulose acetate acetone solution, add 0.7g of dibutyl phthalate DBP and 0.8g of PEG400, and place it on a magnetic stirrer without heating Stir under conditions until the solution is completely dissolved.
[0080] Tablet compression and coating: Install the upper and lower punches, adjust the tablet weight, and adjust the punching pressure to 75N, fill in 250mg of tablet core granules and press into tablets. Put the tablet cores in the coating pan, coat with the pre...
Embodiment 3
[0082] Pass 25mg of lorcaserin hydrochloride, 110mg of mannitol, 8mg of polyvinylpyrrolidone, and 120mg of microcrystalline cellulose through an 80-mesh sieve, weigh them according to the prescription amount, mix them by equal addition method, add 300mg of 75% ethanol solution to make a soft material, and use Granulate with a 20-mesh sieve, dry at 45°C for 6 hours, add 6g of magnesium stearate and mix well.
[0083] Preparation of coating solution: Dissolve 15g of cellulose acetate in 500ml of acetone to prepare a 3% cellulose acetate acetone solution, continue to add 5g of dibutyl phthalate DBP and 1.2g of PEG400, place on a magnetic stirrer Stir without heating until the solution is completely dissolved.
[0084] Tablet compression and coating: install the upper and lower punches, adjust the tablet weight, and adjust the punching pressure to 80N, fill in 250mg of tablet core granules and press into tablets. Put the tablet cores in the coating pan, coat with the prepared coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com