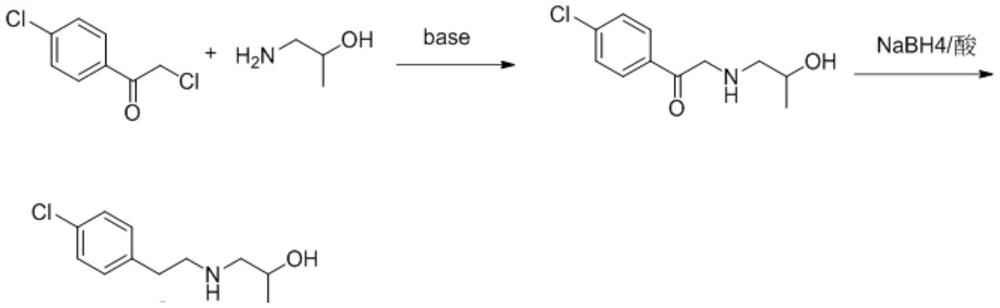
A kind of preparation method of greencaserin key intermediate I
A green caserin, intermediate technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of amino hydroxy compounds, etc., can solve the problem of boron trifluoride ether complex with high toxicity, unsuitable for industrial applications, and environmental pollution. Large and other problems, to achieve the effect of low cost, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of embodiment of the preparation method of green caserin key intermediate I of the present invention, comprises the following steps:
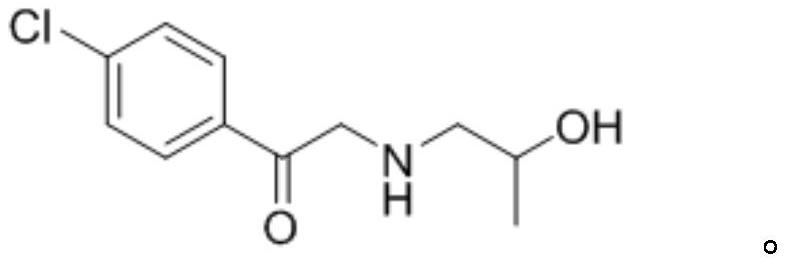
[0035] (1) Preparation of 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethan-1-one
[0036] Put 2,4'-dichloroacetophenone (10g, 52.9mmol), isopropanolamine (4.77g, 63.5mmol) and sodium carbonate (5.6g, 52.9mmol) into the reaction flask at room temperature, add 50ml of ethanol , stirred and heated up to 60°C for 8 hours; distilled off ethanol under reduced pressure, added water and dichloromethane to extract and separate liquids, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried under reduced pressure to obtain a crude product. Add 30ml of petroleum ether into it, beat at 5-10°C for 30 minutes, filter and dry to obtain 11g of solid, which is 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethane-1 - ketone, the yield is 92%;
[0037] After testing, 1 H NMR ...
Embodiment 2
[0042] (1) Preparation of 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethan-1-one
[0043]Put 2,4'-dichloroacetophenone (10g, 52.9mmol), isopropanolamine (4.77g, 63.5mmol) and triethylamine (8g, 79.4mmol) into the reaction flask at room temperature, add 50ml of ethanol Go in, stir and heat up to 60°C for 8h. Ethanol was distilled off under reduced pressure, water and dichloromethane were added for extraction and liquid separation, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was spin-dried under reduced pressure to obtain a crude product, and 30ml of petroleum ether was added into the mixture at 5-10 Slurry at ℃ for 30 minutes, filter and dry to obtain 10.1 g of solid, which is 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethan-1-one, with a yield of 85% ;
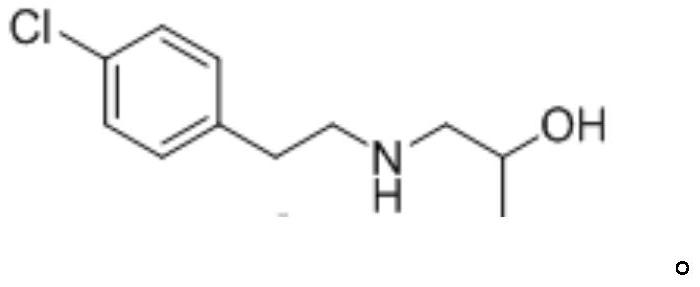
[0044] (2) Preparation of Greencaserin Key Intermediate I
[0045] Dissolve 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethan-1-one (10g, 43.9mmol) in ...
Embodiment 3
[0047] A kind of embodiment of the preparation method of green caserin key intermediate I of the present invention, comprises the following steps:
[0048] (1) Preparation of 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethan-1-one
[0049] At room temperature, 2,4'-dichloroacetophenone (10g, 52.9mmol), isopropanolamine (11.92g, 158.7mmol) and sodium bicarbonate (8.88g, 105.8mmol) were put into the reaction flask, and 50mlN -Methylpyrrolidone, stirred and heated to 120°C for 1 hour; N-methylpyrrolidone was distilled off under reduced pressure, water and dichloromethane were added to extract and separate the liquid, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, The filtrate was spin-dried under reduced pressure to obtain the crude product, and 30ml of petroleum ether was added into it, and it was beaten for 30 minutes at 5-10°C, filtered, and dried to obtain 10.2g of solid, which was 1-(4-chlorophenyl)-2-((2-hydroxy Propyl)am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com