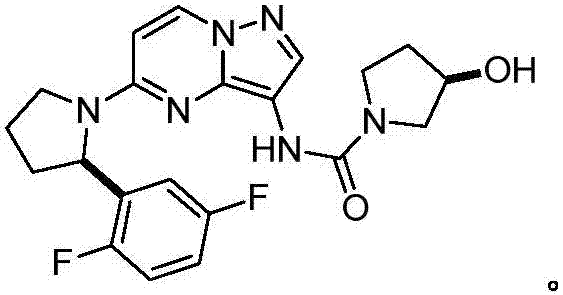
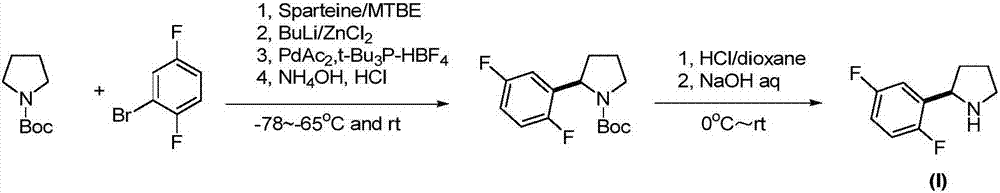
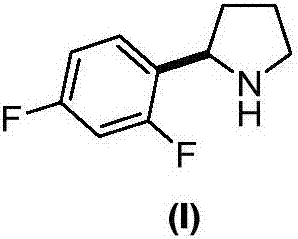
Method for preparing lapatinib intermediate
A technology of latratinib and intermediates, which is applied in the field of preparation of latratinib intermediates, can solve problems such as unfavorable industrial production, reduced atom economy of preparation process, numerous steps, etc., and achieves improved reaction yield and operational feasibility. controllability, reduction of precious metal catalysts and other expensive reagents, and the effect of promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under nitrogen atmosphere, add (7aR,10R,12S)-10-(1-benzotriazolyl)-12-phenyl-7a,8,9,10-tetrahydro-12H-naphthalene to a dry reaction flask Phenol[1,2-e]pyrrolo[2,1-b][1,3]oxazine(II) (4.2g, 10mmol) and 50mL of dry tetrahydrofuran were cooled to -30°C, and 2,5 - Difluorophenylmagnesium(III) bromide (3.2 g, 15 mmol) in THF 30 mL. Keep the temperature at -25~-10°C, stir and react for 2-3 hours, and TLC detects that the reaction is complete. Quench the reaction with 25 mL of saturated ammonium chloride, extract three times with dichloromethane, combine the organic phases, wash with 5% sodium hydroxide solution and saturated brine successively, and dry over anhydrous magnesium sulfate. Concentrate, and recrystallize the residue with ethanol to obtain off-white solid (7aR,10R,12S)-10-(2,5-difluorophenyl)-12-phenyl-7a,8,9,10-tetrahydro- 12H-naphthol[1,2-e]pyrrolo[2,1-b][1,3]oxazine (IV) 3.7g, yield 89.6%, FAB-MS m / z: 414[M+H ] + .
Embodiment 2
[0030] Under nitrogen atmosphere, add (7aR,10R,12S)-10-(2,5-difluorophenyl)-12-phenyl-7a,8,9,10-tetrahydro-12H-naphthalene to a dry reaction flask Phenol[1,2-e]pyrrolo[2,1-b][1,3]oxazine(IV) (3.0g, 7.26mmol) and 40mL of dry diethyl ether, cooled to 0°C, added lithium aluminum hydride (0.41g, 10.9mmol), keep the temperature and stir the reaction for 1 hour, and TLC detects that the reaction is complete. Add 20 mL of pure water to quench the reaction, extract three times with ethyl acetate, combine the organic phases, wash with saturated ammonium chloride aqueous solution and 10% sodium chloride aqueous solution successively, and dry over anhydrous sodium sulfate. Concentrate, and recrystallize the residue with ethyl acetate to obtain off-white solid 1-[(S)-[(2R)-2-(2,5-difluorophenyl)-1-tetrahydropyrrolidinyl]phenyl Methyl]-2-naphthol (V) 2.6g, yield 86.3%, FAB-MS m / z: 416[M+H] + .
Embodiment 3
[0032] Add 1-[(S)-[(2R)-2-(2,5-difluorophenyl)-1-tetrahydropyrrolidinyl]phenylmethyl]-2-naphthol ( V) (2.1g, 5mmol), 10% palladium on carbon (Pd / C) (0.32g, 0.3mmol) and 25mL of methanol, hydrogen gas was passed through at room temperature and normal pressure until the hydrogen gas was no longer absorbed, and the hydrogen gas flow was stopped. The catalyst was recovered by filtration, and the solvent was recovered under reduced pressure to obtain 0.8 g of reddish oil (R)-2-(2,5-difluorophenyl)tetrahydropyrrolidine (I), with a yield of 87.4%; 1H NMR (CDCl 3 )δ7.24(m, 1H), 6.94(m, 1H), 6.85(m, 1H), 4.40(t, J=7.6Hz, 1H), 3.16(m, 1H), 3.04(m, 1H), 2.21-2.30(m, 1H), 1.77-1.95(m, 3H), 1.57-1.67(m, 1H); FAB-MS m / z: 184[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com