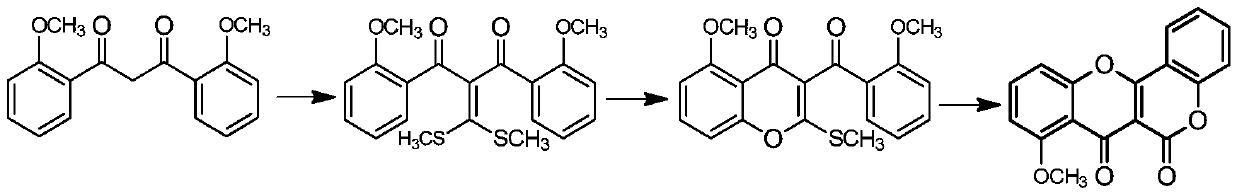
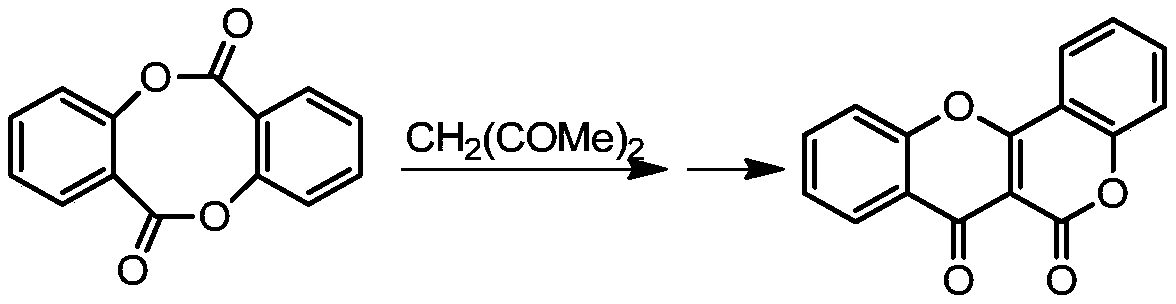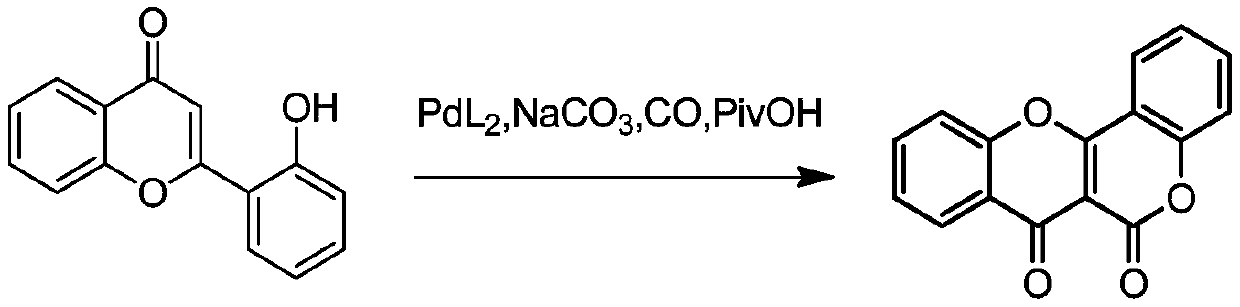Synthesis method of Frutinone compound
A synthesis method and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of less reaction steps, more reaction steps, and difficulty in obtaining, and achieve the effects of mild reaction conditions, cheap raw materials, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The embodiment of the present invention provides a kind of synthetic method of Frutinone compound, comprises the steps:
[0031] (1) in Cs 2 CO 3 Under the action of base, substituted ethyl benzoyl acetate and substituted 2-chlorobenzoyl chloride react to obtain intermediate 1;
[0032] (2) intermediate 1 and boron trihalide carry out hydrolysis reaction, obtain intermediate 2;
[0033] (3) Intermediate 2 is oxidized with potassium persulfate to obtain Frutinone compounds as target products.
[0034] In the present invention, in step (1), the following method is specifically adopted to prepare intermediate 1: alkali, Cs 2 CO 3 Mix evenly with the first solvent, add a mixture of ethyl substituted benzoyl acetate and the first solvent to the mixture at 0-5°C, mix evenly, then add substituted 2-chlorobenzoyl chloride solution, at 0°C After stirring at low temperature for 30-60 minutes, react the mixed solution at 80-130°C for 6-24 hours, and the reaction product is se...
Embodiment 1
[0056] Embodiment 1 of the present invention provides a kind of synthesis of 3A, comprises the following steps:
[0057] (1) Add t-BuONa (1mmol), Cs 2 CO 3 (1mmol) and DMAc (1mL), at 0°C, add ethyl benzoylacetate (1mmol) in DMAc (0.5mL) solution, after stirring for 15min, at 0°C, add 2-chlorobenzoyl chloride (1.2 mmol) in DMAc (1 mL) was added dropwise to the above mixture. After the addition, the resulting mixture was stirred at 0°C for 0.5h, then heated to 110°C, and kept for 8h. After the reaction was completed and cooled to normal temperature, the reaction solution was separated with ethyl acetate and water, the separated aqueous layer was extracted three times with ethyl acetate, the combined extracts were washed with brine, and washed with anhydrous Na 2 SO 4 Drying and concentration under reduced pressure afforded Intermediate 1A.
[0058] (2) Add Intermediate 1A (1mmol) and 20ml of dichloromethane into a 50ml single-necked bottle, stir and mix evenly, add BBr drop...
Embodiment 2
[0061] Embodiment 2 of the present invention provides a kind of synthesis of 3B, comprises the following steps:
[0062] (1) Add t-BuONa (1mmol), Cs 2 CO 3 (1mmol) and DMAc (1mL), at 0°C, add ethyl benzoylacetate (1mmol) in DMAc (0.5mL) solution, after stirring for 15min, at 0°C, add 2,3-dichlorobenzyl A solution of acid chloride (1.2 mmol) in DMAc (1 mL) was added dropwise to the above mixture. After the addition, the resulting mixture was stirred at 0°C for 0.5h, then heated to 110°C, and kept for 8h. After the reaction was completed and cooled to normal temperature, the reaction solution was separated with ethyl acetate and water, the separated aqueous layer was extracted three times with ethyl acetate, the combined extracts were washed with brine, and washed with anhydrous Na 2 SO 4 Drying and concentration under reduced pressure afforded Intermediate 1B.
[0063] (2) Add intermediate 1B (1mmol) and 20ml of dichloromethane into a 50ml single-necked bottle, stir and mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com