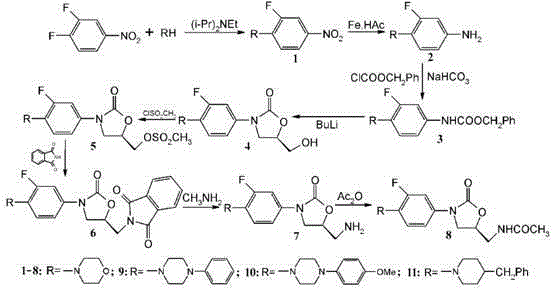
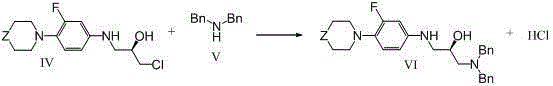The preparation method of 3-substituted phenyl-5-aminomethyl oxazolidin-2-one
A technology of aminomethyl oxazolidine and phenyl, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of inability to adapt to large-scale industrial production, harsh reaction conditions, many reaction steps, etc., and achieves improved safety production coefficient and synthesis efficiency. High, few-step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of 3-substituted phenyl-5-aminomethyloxazolidin-2-one.
[0042] (1)
[0043]Add 39.2 g of compound (II) of 3-fluoro-4-morpholinoaniline, 31.5 g of R-epichlorohydrin (III) and 680 ml of ethanol into a 1000 ml four-neck flask. Heating to 50°C, stirring and reacting at 50-55°C for 24 hours. Cool to about 30°C for the next reaction.
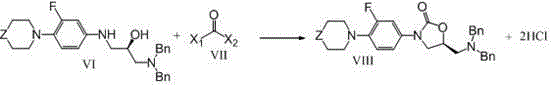
[0044] (2)
[0045] Add the ethanol solution obtained in step (1) and 64g of dibenzylamine to a 1000ml four-necked flask, add 26.5g of sodium hydroxide solution (30%) under stirring, raise the temperature to 60°C, and stir for 12 hours. Ethanol was removed under reduced pressure. Cool to room temperature, add 200ml dichloroethane, 400ml water and 70g concentrated hydrochloric acid (36%), stir for 2-3hrs. Cool to about 10°C, filter with suction, and wash the solid with 100ml of dichloroethane and 150ml of water. Combine the filtrate and washings, separate the layers, and separate the organic phase. Add 300ml of d...
Embodiment 2
[0056] The ethanol solvent in the step (1) of Example 1 was replaced with tetrahydrofuran, and then according to the synthesis method of Steps (1)-(3) of Example 1, about 58 g of the target compound VIII was obtained, with a content of more than 99.0%, and a three-step yield of 60.9 %.
Embodiment 3
[0058] In the step (2) of embodiment one, the reaction temperature of the ethanol feed liquid obtained in the step (1) and dibenzylamine is raised to 100° C. in the presence of sodium hydroxide solution, and then according to the step (2), step (3 ) synthetic method to obtain about 55g of target compound VIII, three-step yield 57.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com