Preparation method for hemihydrate lorcaserin hydrochloride
A technology of hemihydrate and aqueous solution, which is applied in the field of preparation of greencaserin hydrochloride hemihydrate, which can solve the problems of low substance content and unqualified ignition residue, and achieve good yield, suitable for industrial production, and stable crystal form Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, preparation green caserin hydrochloride hemihydrate
[0050] The experimental parameter range of this embodiment is a pilot production batch, and the error range of the heating and cooling system in the workshop is ±5°C, which are the parameter range values determined through the accumulation of a large number of experiments.
[0051] 1. Preparation method
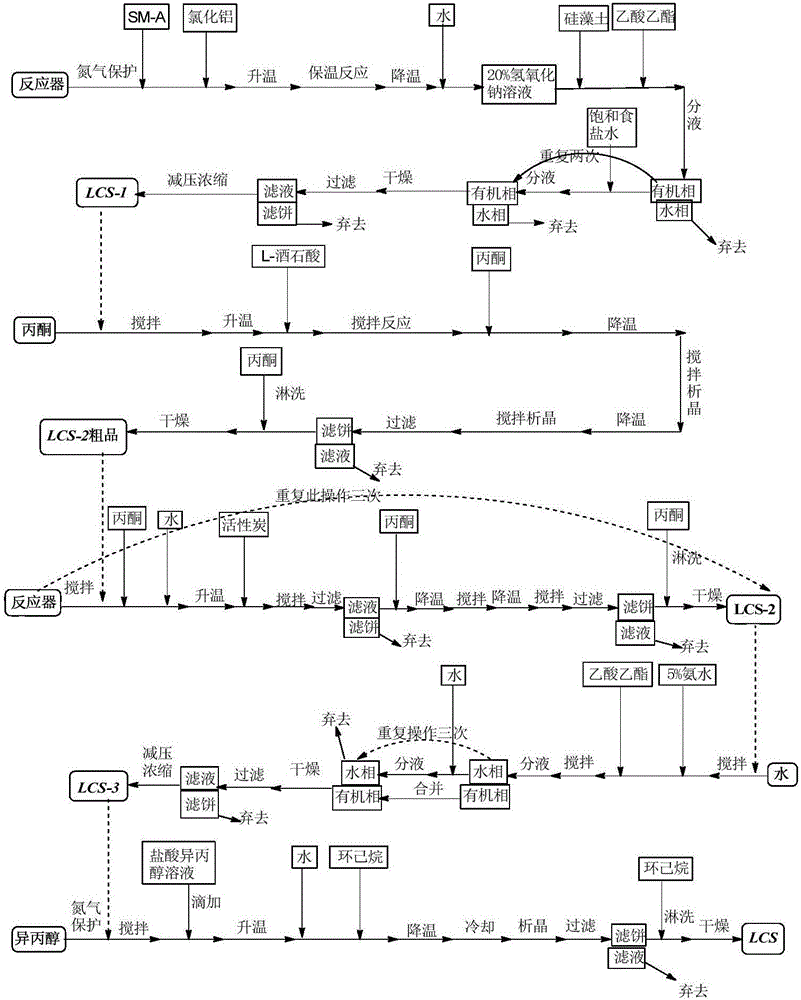
[0052] according to figure 1 Process flow chart shown in prepares green caserin hydrochloride hemihydrate, concrete steps are as follows
[0053] (1) Intermediate (RS)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine (code-named LCS-1, structural formula as shown in formula IV shown) preparation
[0054]
[0055] In a 20L reaction flask, under nitrogen protection, 4050.0g (15.08mol) SM-A (1-[(2-(4-chlorophenyl)ethyl)amino]-2-chloropropane hydrochloride was added successively under stirring, The structural formula is shown in Formula V), 3015.0g (22.61mol) of aluminum chloride, heated to 1...
Embodiment 2
[0163] Embodiment 2, preparation green caserin hydrochloride hemihydrate
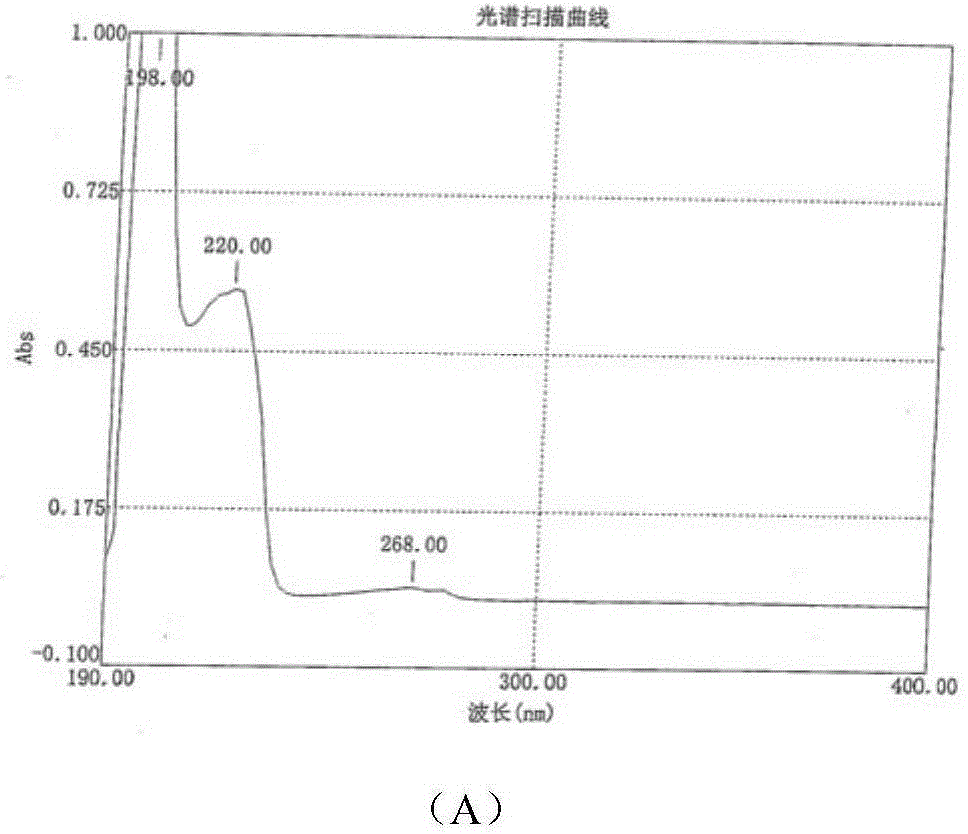
[0164] Prepare green caserin hydrochloride hemihydrate according to the steps in Example 1, only the concentration of ammonia solution in step (3) is replaced by 10% (the mol ratio of LSC-2 and ammonia is the same as in Example 1), the resulting final The HPLC purity of the product was 99.066%.
Embodiment 3
[0165] Embodiment 3, preparation green caserin hydrochloride hemihydrate
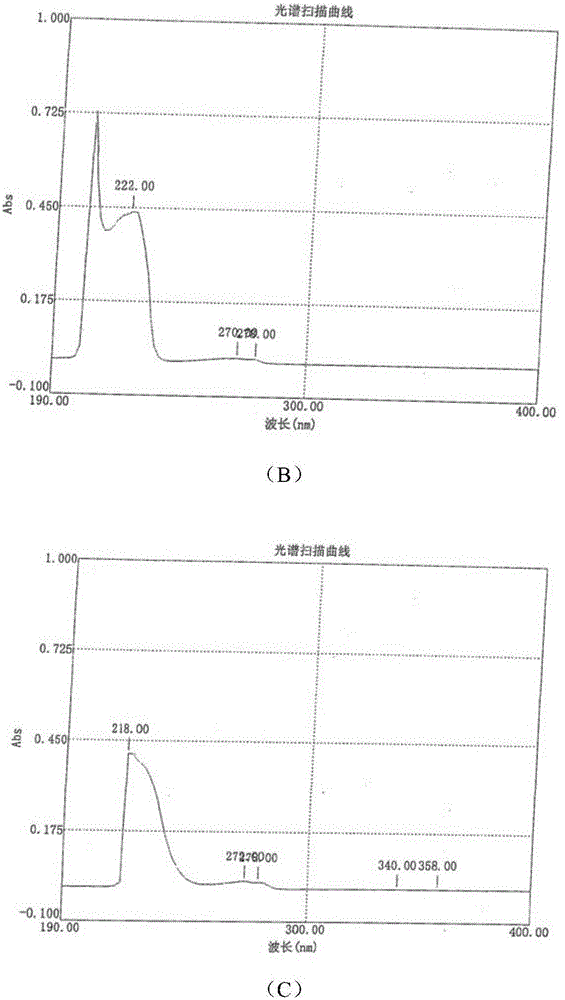
[0166] Green caserin hydrochloride hemihydrate was prepared according to the steps in Example 1, only the isopropanol in step (4) was replaced with 1,4-dioxane, and the HPLC purity of the final product obtained was 99.116%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com