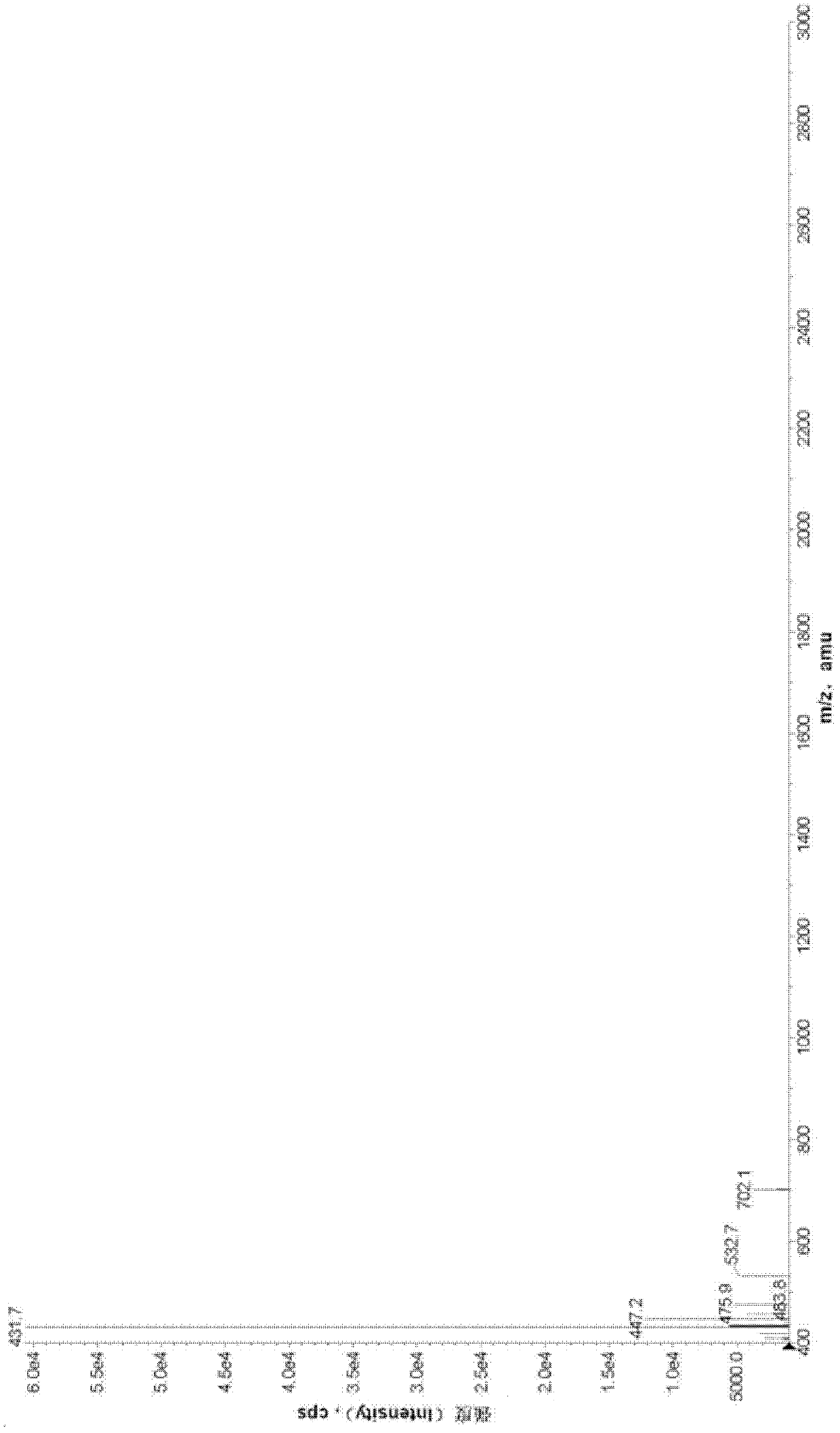
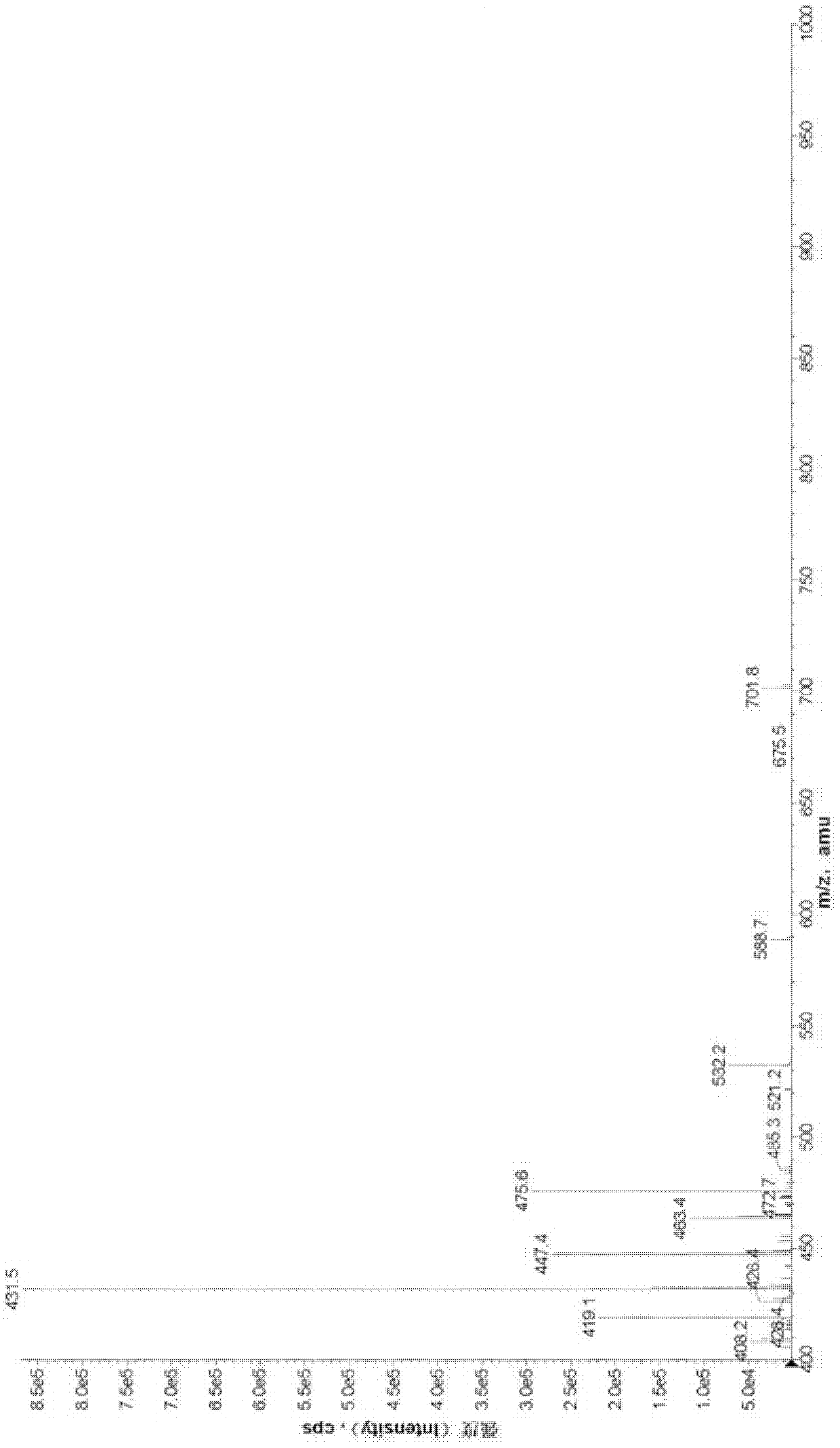
Bilobalide injection and preparation method thereof
A technology of ginkgolide and injection, which is applied in the field of preparation of traditional Chinese medicine preparations, can solve the problems of not forming a drug quality control system, uncertain ratio of ginkgolide, and low content, so as to avoid unqualified clarity and insoluble particles, The effect of solving potential safety hazards and stabilizing the proportion of ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] prescription:
[0200]
[0201] Take glycerin and 80% ethanol in total according to the prescription, mix well, add ginkgolide, stir to dissolve, add ethanol to the full amount, stir evenly, adjust the pH to 3.2-3.8 with 1-10% hydrochloric acid, after the intermediate is qualified, Filter until clear, potting and sterilizing.
Embodiment 2
[0203] prescription:
[0204]
[0205] Take glycerin and 80% ethanol according to the prescription, mix well, add ginkgolide, stir to dissolve, add ethanol to the full amount, stir evenly, adjust the pH to 3.2-3.8 with 5-10% citric acid, the intermediate is qualified Afterwards, filter until clear, potting and sterilizing.
Embodiment 3
[0207] prescription:
[0208]
[0209] Take glycerin and 80% ethanol according to the prescription, mix well, add ginkgolide, stir to dissolve, add ethanol to the full amount, stir evenly, adjust the pH to 3.2-3.8 with 5-10% citric acid, the intermediate is qualified Afterwards, filter until clear, potting and sterilizing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com