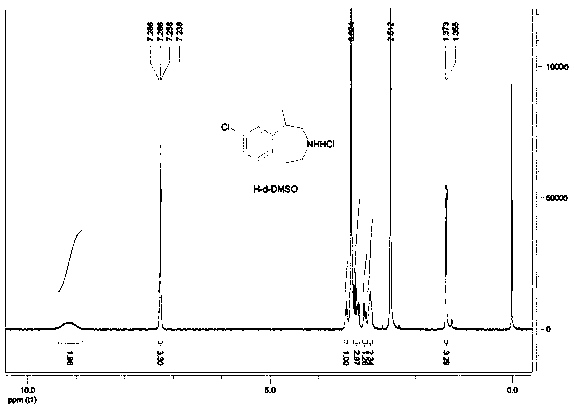
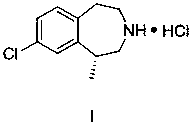
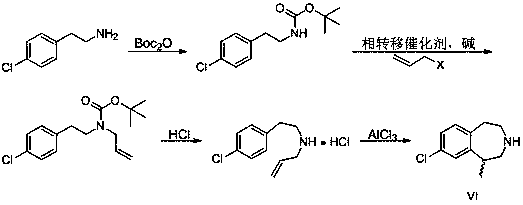
Synthesis process of weight-reducing drug lorcaserin hydrochloride intermediate
A technology of chlorcaserin hydrochloride and synthesis process, which is applied in the directions of drug combination, active ingredients of heterocyclic compounds, metabolic diseases, etc., can solve the problems of high reaction temperature, many impurities, long time, etc., and achieves the simple operation of the synthesis process and the synthesis of The effect of simple route and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0071] The technical solutions of the present invention will be described in detail below in conjunction with specific embodiments.
[0072] Step 1: Acetylation
[0073] Add 7.4kg of acetic anhydride to the 50L reaction kettle successively, then add 7.5kg of p-chlorophenethylamine dropwise, start stirring and heating, raise the temperature to 50-60°C for 2 hours, take samples and send them to the central control of HPLC; Excess acetic anhydride and by-product acetic acid were removed; 20kg of toluene and water were added for washing, and the organic phase directly entered the next step of allyl substitution reaction after standing for separation.
[0074] Step Two: Replace
[0075] Add 3.0kg of tetrabutylammonium bromide, 11.4kg of allyl bromide, 19.6kg of potassium carbonate and 26.5kg of potassium hydroxide into a 100L reactor, start stirring and raise the temperature to 50°C to 60°C for 10 to 12 hours; filter , the filter cake was washed with a small amount of toluene; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com