Hydrochloric lorcaserin tablets and preparing method thereof
A technology of lorcaserin hydrochloride and tablets, which is applied in the fields of pill delivery, metabolic diseases, active ingredients of heterocyclic compounds, etc., and can solve problems such as cumbersome preparation process, unfavorable treatment of obese patients, easy to overdose, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
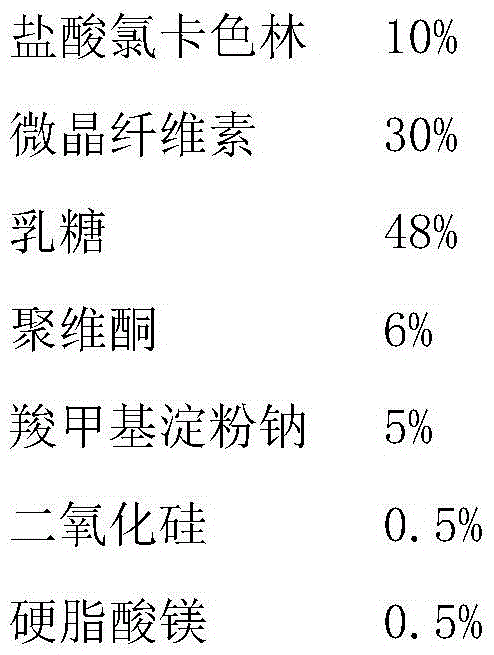
[0028]
[0029] Preparation process: pass the main drug, microcrystalline cellulose, lactose, povidone and sodium carboxymethyl starch through an 80-mesh sieve, then weigh each material except the lubricant, and deliver the auxiliary materials in the same amount as the main drug in turn. Add and mix evenly; add lubricant and mix evenly, and use powder direct compression technology to compress the tablet, so that the tablet weight is 80-250mg, and the hardness is 20-120N.
Embodiment 2
[0031]
[0032] Preparation process: pass the main drug, microcrystalline cellulose, lactose, povidone and low-substituted hydroxypropyl cellulose through an 80-mesh sieve, then weigh each material except the lubricant, and mix the auxiliary materials with the main drug, etc. Add the amount and mix evenly; add lubricant and mix evenly, and use powder direct compression technology to compress the tablet, so that the tablet weight is 80-250mg, and the hardness is 20-120N.
Embodiment 3
[0034]
[0035] Preparation process: pass the main drug, microcrystalline cellulose, lactose, povidone and crospovidone through an 80-mesh sieve respectively, then weigh each material except the lubricant, and deliver the auxiliary materials in the same amount as the main drug in turn. Add and mix evenly; add lubricant and mix evenly, and use powder direct compression technology to compress the tablet, so that the tablet weight is 80-250mg, and the hardness is 20-120N. Example 4
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com