Synthesis method of amino-protecting glycine dipeptidase derivant
A technology of glycine dipeptide and fluorenylmethoxycarbonyl glycine dipeptide, which is applied in the direction of peptides, etc., can solve the problems that product separation is not suitable for large-scale production, product yield and purity are low, and achieve the effect of environmental friendliness and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
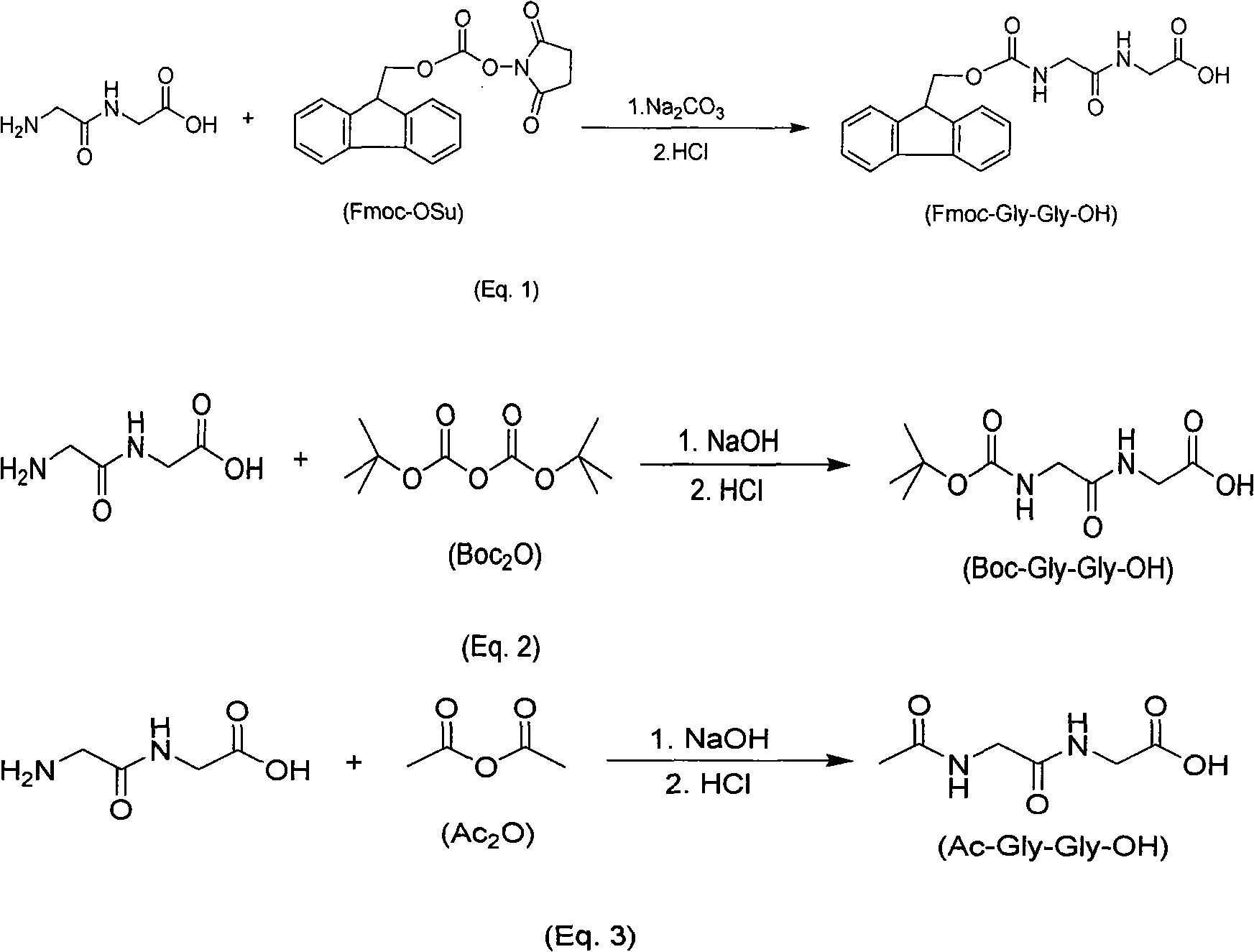
[0025] Dissolve 6.1 grams (0.050 moles) of glycine dipeptide in 63 mL of 10% sodium carbonate solution, stir to dissolve the glycine dipeptide, and add dropwise Fmoc-ONSu (16.8 grams, 0.05 mol) solution, the dropwise addition was completed in 30 minutes. After the dropwise addition, the reaction was stirred at 30° C. for 2 hours, diluted with about 50 mL of water, and the reaction solution was extracted with toluene (80 mL), and the resulting aqueous phase was acidified to pH = 2, and then extracted with ethyl acetate (100 mL), the obtained organic phase was washed with water, and the organic phase was concentrated to remove the ethyl acetate solvent, and a white solid was precipitated. After filtering and drying, 16.1 g of the product Fmoc-Gly-Gly-OH was obtained, with a yield of 91%.
Embodiment 2
[0027] Dissolve 6.1 grams (0.05 moles) of glycine dipeptide in 79 mL of 10% potassium carbonate solution, stir to dissolve the glycine dipeptide, and add dropwise Fmoc-ONSu (17.7 grams, 0.0525 mol) solution, the dropwise addition was completed in 30 minutes. After the dropwise addition, the reaction was stirred at 30° C. for 2 hours, diluted with about 50 mL of water, and the reaction solution was extracted with toluene (80 mL), and the resulting aqueous phase was acidified to pH = 2, and then extracted with ethyl acetate (100 mL), the obtained organic phase was washed with water, and the organic phase was concentrated to remove the ethyl acetate solvent, and a white solid was precipitated. After filtering and drying, 16.5 g of the product Fmoc-Gly-Gly-OH was obtained, with a yield of 93%.
Embodiment 3
[0029] Dissolve 6.1 g (0.050 moles) of glycine dipeptide in 63 mL of 10% sodium carbonate solution, stir to dissolve the glycine dipeptide, and add Boc dissolved in methanol (60 mL) dropwise at 20°C 2 O (10.9 g, 0.05 mol) solution, the dropwise addition was completed in 30 minutes. After the dropwise addition was completed, the reaction was stirred at 30° C. for 2 hours, and the reaction solution was extracted with methyl tert-butyl ether (80 mL). Acidify with hydrochloric acid to pH = 3, and then extract with ethyl acetate (100 mL). The obtained organic phase is washed with water, and then the organic phase is concentrated to remove the ethyl acetate solvent, and a white solid is precipitated. After filtering and drying, 9.29 g of the product Boc-Gly-Gly-OH was obtained, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com