Production method of N-protection pipradrol
A production method and a technology for piperidinol, which are applied in the production field of N-protected piperidinol, can solve the problems of low efficiency, high industrialization cost, harsh reaction conditions and the like, and achieve the effects of high efficiency, simplicity and good industrial application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
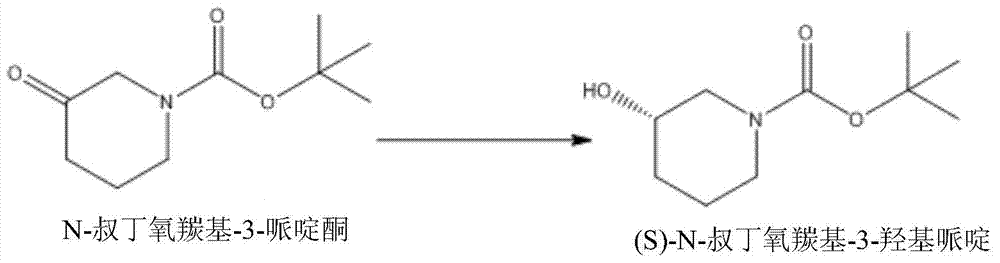
[0049] Embodiment 1, N-tert-butoxycarbonyl-3-piperidone is used as a substrate for enzyme screening
[0050] Synthesize the gene sequences of 19 alcohol dehydrogenases and ADH-A from different sources and design restriction enzyme sites at both ends of the genes, and then subclone them into the corresponding sites of the vector pET24a (purchased from Novagen). The obtained recombinant plasmid was transformed into Escherichia coli host cell BL21(DE3) for expression.
[0051] Weigh 0.4g of N-tert-butoxycarbonyl-3-piperidone and add them to 19 250ml shake flasks, then add 2ml of isopropanol and 10ml of water, adjust the pH to about 8.0 with ammonia water, and adjust the pH according to the final concentration of 40000U / L (enzyme activity assay scheme refers to literature Levin I, Meiri G, Peretz M, et al. The ternary complex of Pseudomonas aeruginosa alcohol dehydrogenase with NADH and ethylene glycol[J]. Protein science, 2004, 13(6): 1547-1556 The method in ) was added to ADH-...
Embodiment 2
[0057] Embodiment 2, N-tert-butoxycarbonyl-3-piperidone is the conversion of substrate
[0058] 2.1. Transformation of cpsADH broken solution
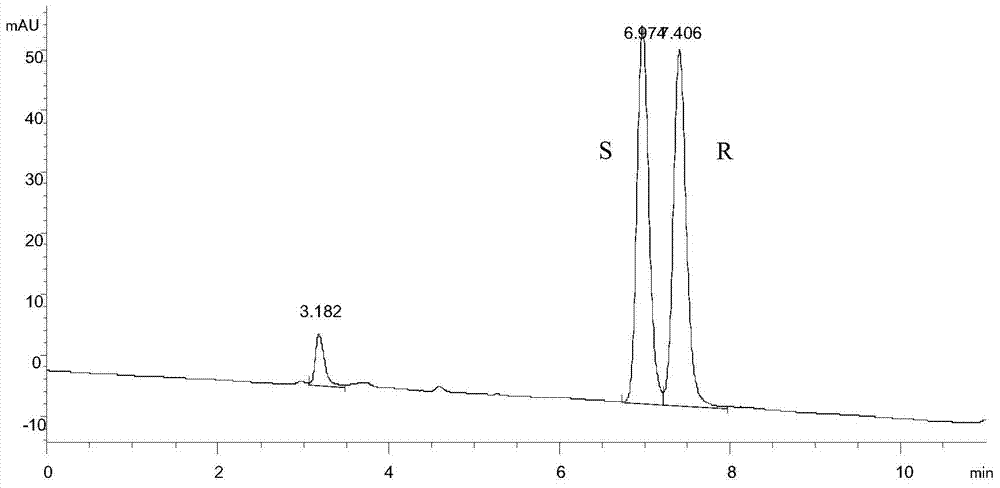
[0059] Weigh 100g of the substrate N-tert-butoxycarbonyl-3-piperidone, 200ml of isopropanol, add it to a 2000ml fermenter, add 200ml of water, adjust the pH to about 8.0 with ammonia water, and then add the enzyme according to the final concentration of 40000U / L Liquid ADH-A, according to the final concentration of 1g bacteria / L (converted according to the weight of the bacteria before crushing), add cpsADH crushing liquid, set the volume to 1000ml volume, adjust the pH to 8.0 with ammonia water, add 0.03g / L NAD + The reaction was started, and air was introduced at 0.5vvm, and the conversion result was detected by GC method after 3 hours of conversion, and the conversion rate reached 98.9%; the ee value of the product detected by chiral HPLC was greater than 99.5%.
[0060] 2.2. Transformation of cpsADH cells
[0061] Weigh 100g of...
Embodiment 3
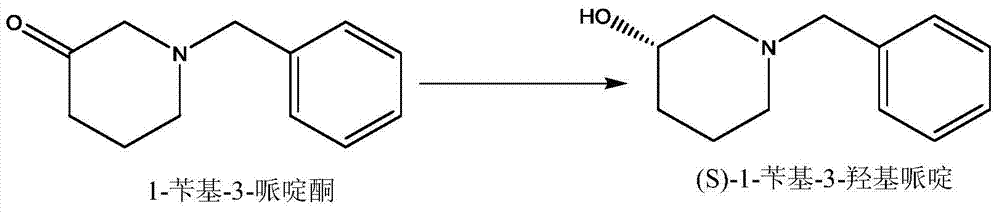
[0066] Embodiment 3,1-benzyl-3-piperidone is the conversion of substrate
[0067] 3.1. Transformation of cpsADH cells
[0068] Weigh 100g of the substrate 1-benzyl-3-piperidone, 200ml of isopropanol, add it to a 2000ml fermenter, add 200ml of water, adjust the pH to about 8.0 with ammonia water, and then add the enzyme solution ADH according to the final concentration of 40000U / L -A, add cpsADH cells at a final concentration of 1g cells / L, set the volume to 1000ml, adjust the pH to 8.0 with ammonia water, add 0.03g / L NAD at 40°C and rotate at 500rpm + The reaction was started, and air was introduced at 0.5vvm, and the conversion result was detected by GC method after 5 hours of conversion, and the conversion rate reached 99.0%; the ee value of the product detected by chiral HPLC was greater than 99.5%.
[0069] 3.2. Transformation of cmADHmut cells
[0070] Weigh 100g of the substrate 1-benzyl-3-piperidone, 200ml of isopropanol, add it to a 2000ml fermenter, add 200ml of wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com