Method for preparing o-alkoxyphenol from epoxy cyclohexane
A technology for o-alkoxyphenol and epoxy cyclohexane, applied in the field of preparing o-alkoxyphenol, can solve the problems of many side reactions, low yield, poor selectivity, etc., and achieves the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
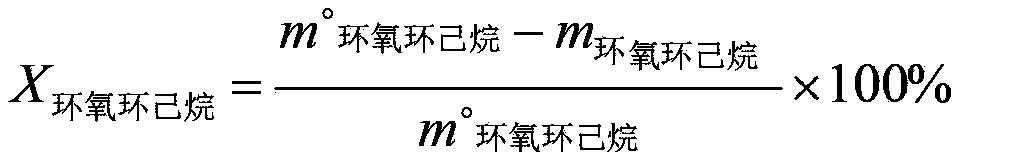
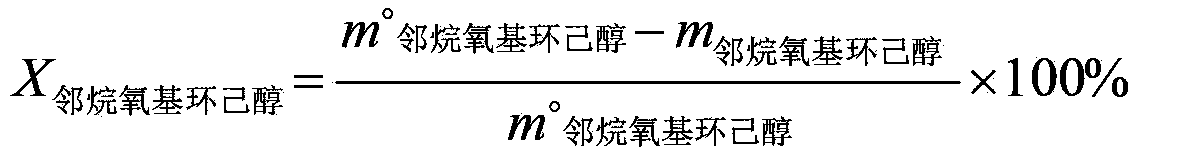
[0042] Under alcoholysis conditions, contact epoxycyclohexane and methanol with an alcoholysis catalyst to separate and obtain o-methoxycyclohexanol; wherein, the alcoholysis conditions include: the temperature is 60°C, the alcoholysis catalyst and epoxycyclohexane The mass ratio of alkane is 0.05:1, the alcoholysis catalyst is sodium bisulfate, and the molar ratio of epoxycyclohexane to methanol is 1:3; the selectivity of o-methoxycyclohexanol is 96%, and the conversion of epoxycyclohexane The rate is 98.1%.
Embodiment 2
[0044] Under alcoholysis conditions, contact epoxycyclohexane and ethanol with an alcoholysis catalyst to separate and obtain o-ethoxycyclohexanol; wherein, the alcoholysis conditions include: the temperature is 80°C, the alcoholysis catalyst and epoxycyclohexane The mass ratio of alkane is 0.01:1, the alcoholysis catalyst is phosphoric acid, and the molar ratio of epoxycyclohexane to ethanol is 1:20; the selectivity of o-ethoxycyclohexanol is 96%, and the conversion rate of epoxycyclohexane is 99.3% %.
Embodiment 3-6
[0046] Prepare other o-alkoxy cyclohexanols according to the method of Example 1, the difference is that the alcohols used are n-propanol, n-butanol, cyclohexanol, n-octanol, and finally prepare o-n-propoxy cyclohexanol Hexanol, o-n-butoxycyclohexanol, o-cyclohexyloxycyclohexanol, o-n-octyloxycyclohexanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com