Chiral preparation method for N-t-butyloxycarboryl-(4S)-(p-phenyl phenyl methyl)-4-amino-(2R)-methylbutyric acid
A technology of phenylphenylmethyl and tert-butoxycarbonyl is applied in the field of chiral preparation of N-tert-butoxycarbonyl---4-amino-methylbutyric acid, which can solve the problem of unsuitable industrial production and price Expensive, increased reaction costs, etc., to achieve the effect of easy to obtain reagents, simple reaction conditions, and guaranteed purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
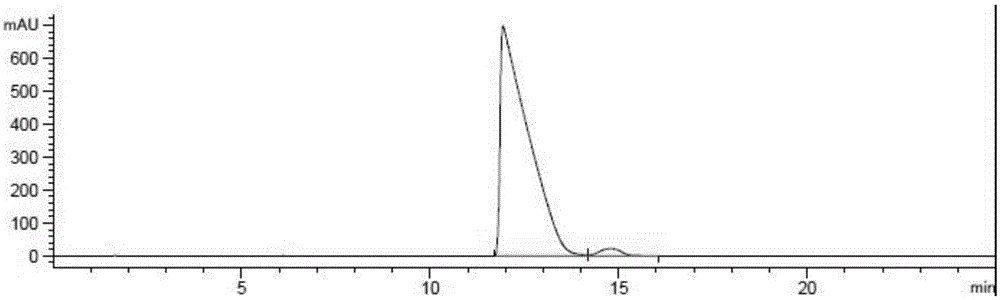
[0030] Get compound (1) (20.0g, 1.0 equivalent) and dissolve in ethanol (50ml), add (S)-phenethylamine (5.9g, 1.0 equivalent), 10%Pd / C (2.0g), in 4.0bar Under hydrogen pressure, the reaction solution was placed in an oil bath at 40°C, heated and stirred for 2.5 hours, then cooled to room temperature, the system was a gray suspension, filtered directly to obtain an off-white solid, which was placed in a reaction flask, and water (20ml) was added , dichloromethane (80ml), hydrochloric acid (6mol / L, 10ml), stirred at room temperature for 1 hour, filtered to remove Pd / C, then separated the system, collected the dichloromethane layer, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure The solvent was removed to obtain 20.1 g of a white solid. HPLC detection showed that the diastereomer ratio was (2-a):(2-b)=97.62:2.38.
[0031] Purified by recrystallization with 100ml of ethyl acetate / heptane (1:4) to obtain 18.5g of white solid. HPLC...
Embodiment 2
[0035]
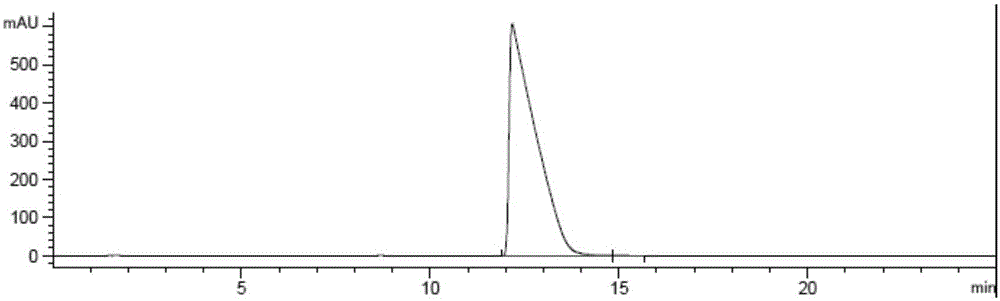
[0036] Get compound (1) (500g, 1.0 equivalent) and dissolve in ethanol (1300ml), add (S)-phenethylamine (148g, 1.0 equivalent), 10%Pd / C (50g), under the hydrogen pressure of 4.0bar The reaction solution was heated and stirred in a 40°C oil bath for 2.5 hours, then cooled to room temperature, the system was a gray suspension, filtered directly to obtain an off-white solid, which was placed in a reaction bottle, and water (500ml), dichloro Methane (2000ml), hydrochloric acid (6mol / L, 250ml), stirred at room temperature for 1 hour, filtered to remove Pd / C, then separated the system, collected the dichloromethane layer, dried over anhydrous sodium sulfate, filtered, evaporated the solvent under reduced pressure, 484.8 g of white solid were obtained. HPLC detection showed that the diastereomer ratio was (2-a):(2-b)=97.69:2.31.
[0037]Purified by recrystallization with 2500ml of ethyl acetate / heptane (1:4) to obtain 452.2g of white solid. HPLC detection showed that th...
Embodiment 3
[0039]
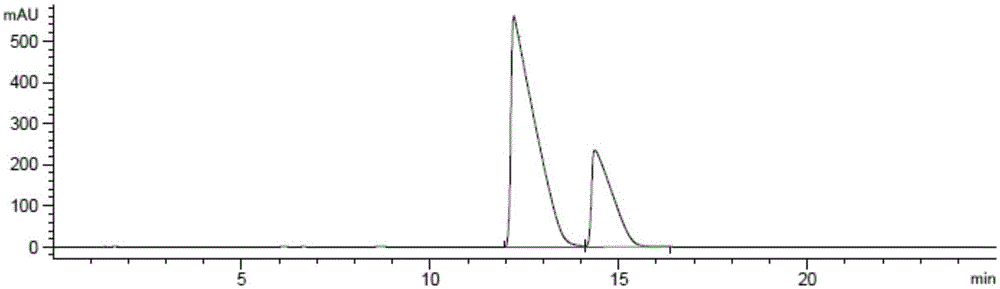
[0040] Dissolve compound (1) (20.0 g) in ethanol (50 ml), add 10% Pd / C (2.0 g), and place the reaction solution in a 40° C. oil bath with stirring for 2 hours under a hydrogen pressure of 3.5 bar, and then After cooling to room temperature, Pd / C was removed by filtration, and the solvent was evaporated under reduced pressure to obtain compound (2) (21.0 g) as a white solid. HPLC detection, compound (2-a):(2-b)=73.34:26.66.
[0041] The above compound (2) (21.0 g, 1.0 equivalent) was dissolved in 210 ml of ethanol, and (S)-phenethylamine (6.3 g, 1.0 equivalent) was slowly added under stirring at room temperature, and a white solid gradually precipitated out of the system. After stirring for 2 hours, , filtered, and the filter cake was washed with 105ml ethanol. Place the washed filter cake in a reaction flask, add water (20ml), methylene chloride (80ml), hydrochloric acid (6mol / L, 10ml), stir at room temperature for 1 hour, separate the system, and collect the meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com