Method for preparing high-purity palbociclib and reaction intermediate of palbociclib
A reaction and purity technology, applied in the field of preparation of high-purity palbociclib and its reaction intermediate, palbociclib, can solve the problem of high production equipment requirements, harsh reaction conditions, Problems such as low utilization rate, to overcome the low utilization rate of raw materials, strong operability and controllability, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
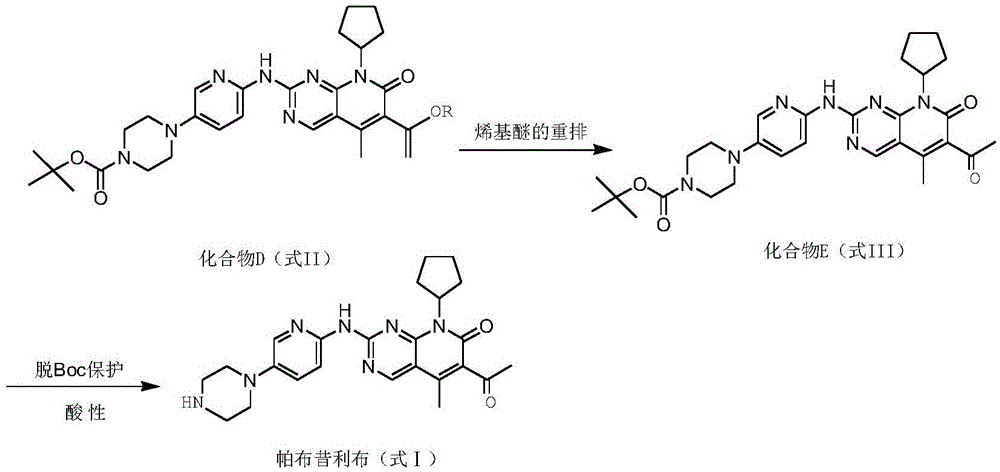
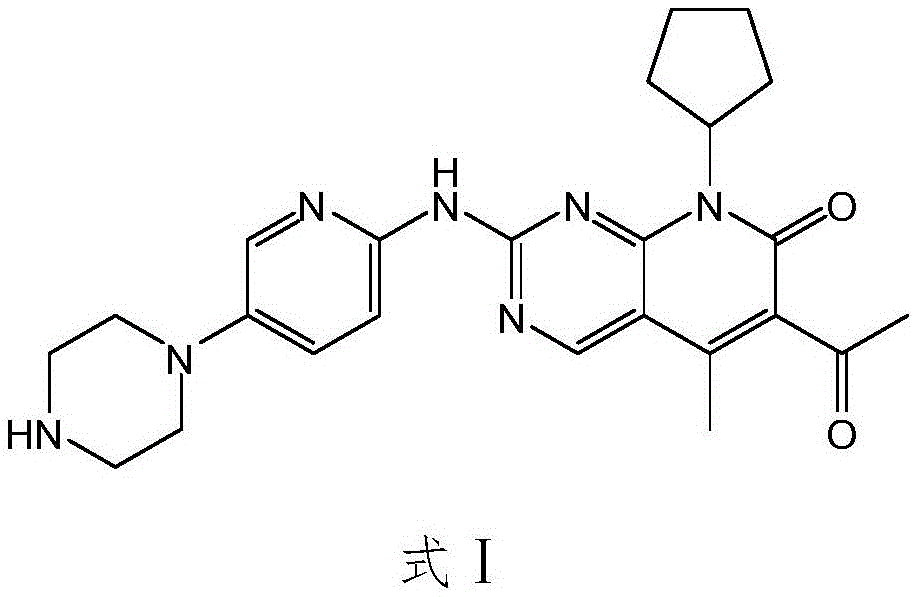
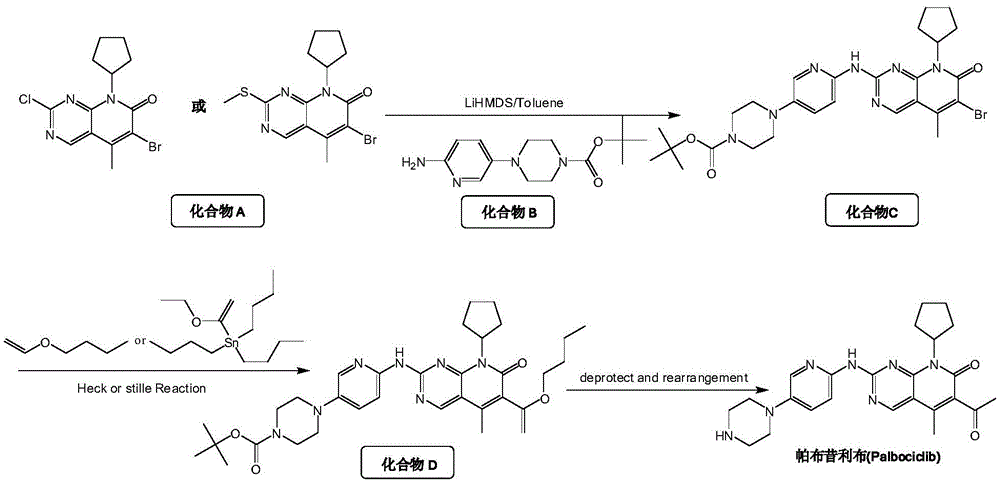
[0056] At room temperature, compound D1(C) was added to the dry reaction flask 30 H 39 N 7 O 4 5.6g, 10mmol, purity ≥99.5%), HCl (7.3g, 200mmol), CH 3 OH (56.1 ml) was slowly mixed, and the reaction was stirred at 80 °C for 12 h. After the reaction was completed, the mixture was cooled and crystallized, and a small amount of CH was added. 3 The mixed solvent of OH and petroleum ether was recrystallized to obtain light yellow solid E. Solid E is then suspended in C 2 H 5In OH (57.0ml), HCl (7.3g, 200mmol) was added dropwise, and the reaction was stirred at 80°C for 6h. After the reaction was completed, NaOH was added to adjust the pH to 11, cooling and crystallization, suction filtration, and drying to obtain pure Pabbu. 3.5 g of corybine free base, yield 78%; mass spectrum (EI): m / z 448 (M+H).
Embodiment 2
[0058] At room temperature, compound D2 (C 33 H 45 N 7 O 4 7.2g, 12mmol, purity ≥99.5%), HCl (12.0g, 600mmol), CH 3 OH (217.0 ml) was slowly mixed, and the reaction was stirred at 60 ° C for 18 h. After the reaction was completed, the mixture was cooled and crystallized, and a small amount of CH was added. 3 The mixed solvent of OH and petroleum ether was recrystallized to obtain yellow solid E. Solid E is then suspended in C 2 H 5 In OH (220.0ml), HCl (12.0g, 600mmol) was added dropwise, and the reaction was stirred at 80°C for 6h. After the reaction was completed, cooling and crystallization, suction filtration, and drying were to obtain pure Palbociclib free base. 4.2 g, 79% yield; mass spectrum (EI): m / z 448 (M+H).
Embodiment 3
[0060] At room temperature, compound D3 (C 31 H 40 ClN 7 O 4 6.1g, 10mmol, purity ≥99.5%), CH 3 COOH (12.0 g, 200 mmol), CH 3 OH (91.4ml) was slowly mixed, and the reaction was stirred at 60°C for 12h. After the reaction was completed, the mixture was cooled and crystallized, and a small amount of CH was added. 3 The mixed solvent of OH and petroleum ether was recrystallized to obtain light yellow solid E. Solid E is then suspended in C 2 H 5 OH (92.0ml) was added dropwise with HCl (7.3g, 200mol), and the reaction was stirred at 80°C for 3h. After the reaction was completed, cooling and crystallization, suction filtration and drying were performed to obtain pure Palbociclib free base. 3.4 g, 76% yield; mass spectrum (EI): m / z 448 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com