Target mitochondrion antioxidant as well as preparation method and application of target mitochondrion antioxidant
A technology of nitrogen oxides and compounds, applied in the field of biochemistry, can solve problems that cannot delay the progress of diseases and cannot be cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 prepares compound I
[0064] one, Synthesis of intermediate 13:
[0065] First, intermediate 13 was synthesized according to the following synthetic route:
[0066]
[0067] Step 1: Synthesis of Compound 5
[0068] Compound 4 (10 g, 37.6 mmol) was dissolved in methanol (150 mL), cooled to 0°C, and thionyl chloride (22 g, 0.19 mmol) was added dropwise thereto.
[0069] Under stirring, the reaction solution was gradually raised to room temperature, and the stirring was continued for 12 h. The reaction solution was concentrated under reduced pressure to obtain compound 5 (11.7 g), with a yield of 98%.
[0070] Step 2: Synthesis of Compound 2
[0071] Compound 1 (6.45 g, 30 mmol) and D-Valine methyl ester hydrochloride (5.53 g, 33 mmol) were dissolved in dichloromethane (200 mL), cooled to 0°C. To the above solution were added 1-hydroxybenzotriazole (4.46 g, 33 mmol), carbodiimide (6.3 g, 33 mmol) and DIEA (8.51 g, 66 mmol).
[0072] The reaction sol...
Embodiment 2
[0149] Embodiment 2 physical and chemical properties are measured
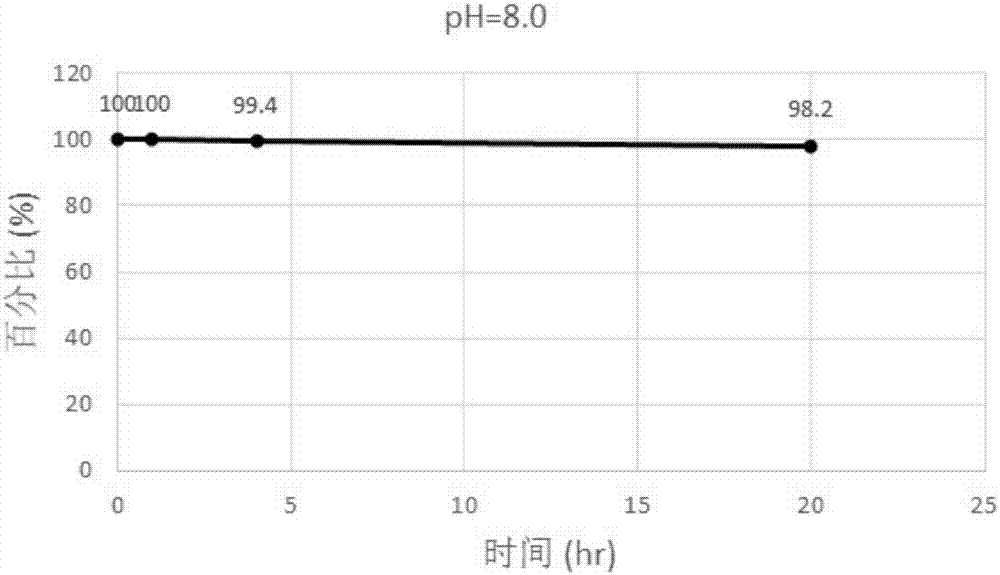
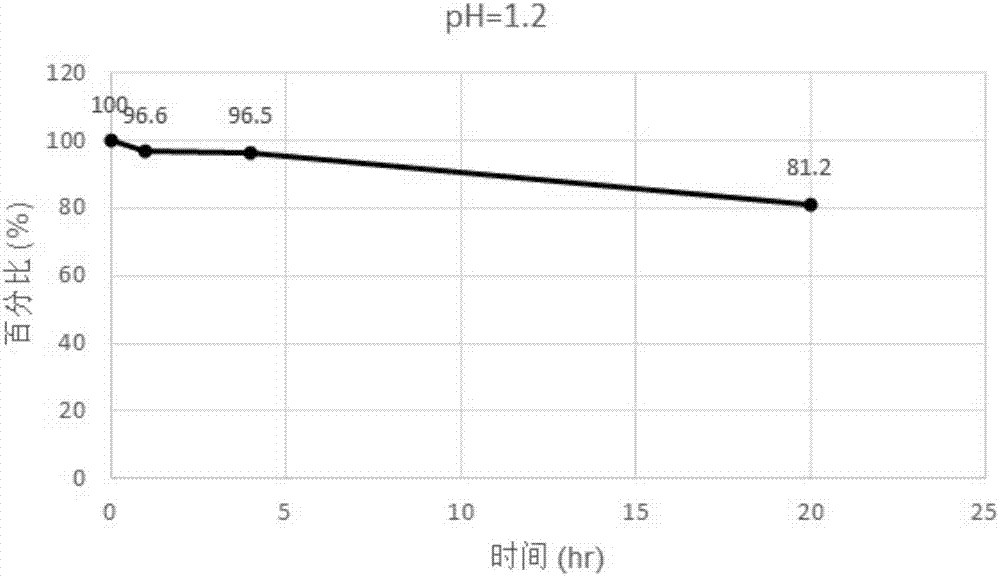
[0150] 1. Investigation of pH stability
[0151] Prepare pH 1.2 and 8.0 buffer solutions as follows:
[0152] pH = 1.2 hydrochloric acid solution: take 765ul of concentrated hydrochloric acid in a volumetric flask, dilute to 100ml, and obtain.
[0153] pH=8.0 Phosphate buffer solution: 0.2mol / L potassium dihydrogen phosphate solution (solution A): take 2.722g of potassium dihydrogen phosphate, add water to dissolve, and dilute to 100ml; 0.2mol / L sodium hydroxide solution (solution B) : Take 800mg of sodium hydroxide, add water to dissolve, and dilute to 100ml; take 25ml of solution A, 23.05ml of solution B, add water to dilute to 100ml, and obtain.
[0154] The incubation was designed and implemented as follows:
[0155] Compound I stock solution preparation: 1 g of Compound I prepared in Example 1 was dissolved in a volumetric flask, and acetonitrile was added to 10 ml to obtain a standard solution with a ...
Embodiment 3
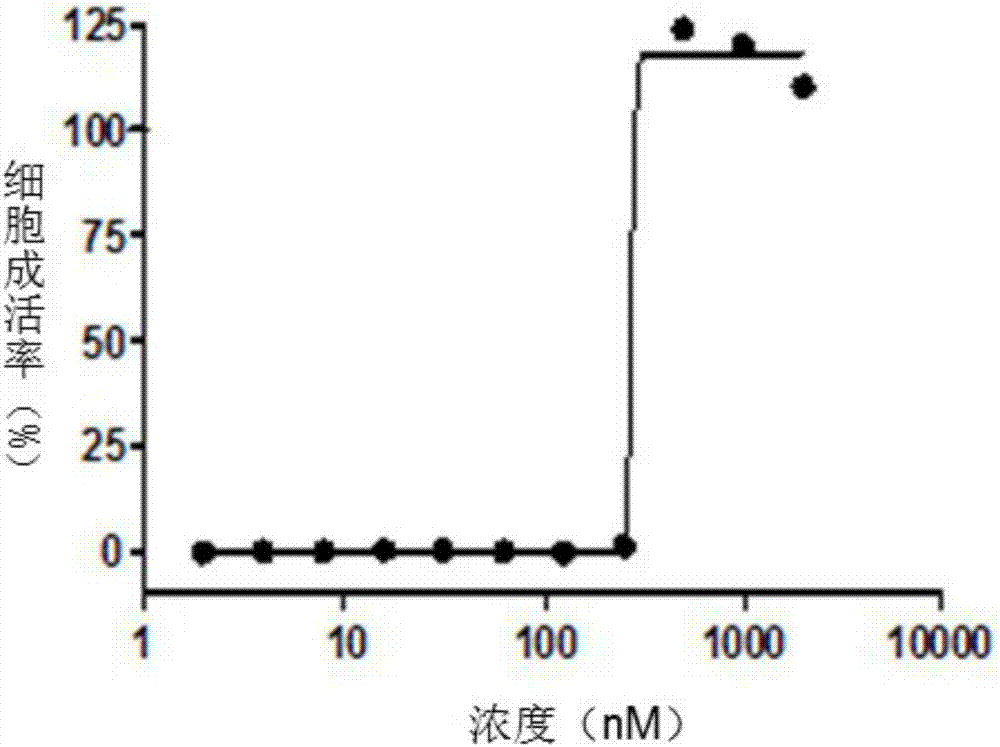
[0162] Example 3 Compound I has inhibitory effect on Erastin-induced HT 1080 cell ferroptosis (Ferroptosis)
[0163] After the diluted cells were added to the 96-well plate for adherent growth, 10 μM Rastin was added to induce the state of ferroptosis (Ferroptosis) in HT1080 cells, and then compound I was added at different concentrations. After 6 wells of each concentration were incubated for 24 h, the cells were incubated with CTG ( CellTiter-Glo) method was used to detect cell viability, and the half maximum effective concentration of compound I was obtained as EC50=275.2nM.
[0164] The experimental results show that compound I can inhibit the ferroptosis induced by Erastin in HT1080 cells, indicating that our compound I can target mitochondria and can eliminate free radicals generated in cells (see image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com