Star-shaped cationic polymer containing dendriform polylysine element and preparation method thereof
A technology of cationic polymers and polylysine units, which is applied in the field of biomedical engineering materials to achieve high reaction efficiency, low raw material consumption, and low toxicity of metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
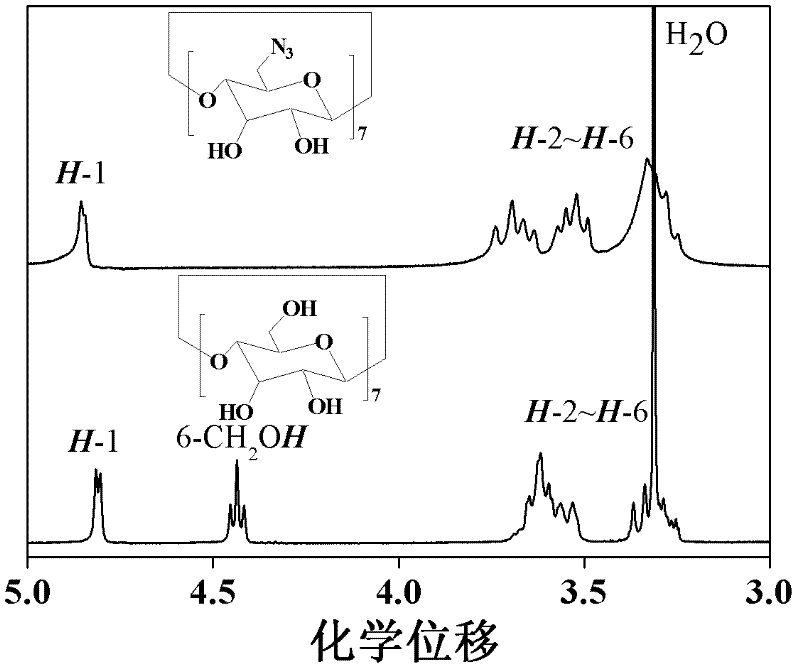
[0055] a. Weigh triphenylphosphine and dissolve it in anhydrous N, N-dimethylformamide (DMF), then add iodine element at room temperature at 25°C, raise the temperature to 55°C; then add dry β-cyclopaste Fine (β-CD), N 2 Reaction under atmosphere for 48 hours; after the reaction, anhydrous N,N-dimethylformamide was removed by distillation under reduced pressure, and sodium methoxide solution was added under ice-water bath conditions; the resulting product was precipitated 7 times in methanol, and then vacuum-dried to obtain seven (6-iodo-6-deoxy)-β-cyclodextrin [β-CD-(I) 7 ]; the molar ratio of the β-cyclodextrin, triphenylphosphine and iodine simple substance is 1: 1.2: 1.4; the anhydrous N, N-dimethylformamide is added with 20 grams of dry Beta-cyclodextrin meter; the molar number of sodium methylate in the sodium methylate solution is 1.5 times of the dry beta-cyclodextrin molar number;
[0056] b. Dissolve the hepta(6-iodo-6-deoxy)-β-cyclodextrin and sodium azide obtaine...
Embodiment 2
[0058] a. Weigh triphenylphosphine and dissolve it in anhydrous N,N-dimethylformamide (DMF), then add iodine element at room temperature at 35°C, and raise the temperature to 70°C; then add dry β-cyclopaste Fine (β-CD), N 2 Reaction under atmosphere for 24 hours; after the reaction, anhydrous N, N-dimethylformamide was distilled off under reduced pressure, and sodium methoxide solution was added under ice-water bath conditions; the resulting product was precipitated 4 times in methanol, and then vacuum-dried to obtain seven (6-iodo-6-deoxy)-β-cyclodextrin [β-CD-(I) 7 ]; the molar ratio of the β-cyclodextrin, triphenylphosphine and iodine simple substance is 1: 1.5: 1.6; the anhydrous N, N-dimethylformamide is added 5 grams of dry Beta-cyclodextrin meter; the molar number of sodium methylate in the sodium methylate solution is 2.0 times of the dry beta-cyclodextrin molar number;
[0059] b. Dissolve the hepta(6-iodo-6-deoxy)-β-cyclodextrin and sodium azide obtained in step a ...
Embodiment 3
[0061] a. Weigh triphenylphosphine and dissolve it in anhydrous N,N-dimethylformamide (DMF), then add iodine element at room temperature at 5°C, and raise the temperature to 60°C; then add dry β-cyclopaste Fine (β-CD), N 2 Reaction under atmosphere for 32 hours; after the reaction, anhydrous N,N-dimethylformamide was removed by distillation under reduced pressure, and sodium methoxide solution was added under ice-water bath conditions; the resulting product was precipitated in methanol for 6 times, and then dried in vacuum to obtain seven (6-iodo-6-deoxy)-β-cyclodextrin [β-CD-(I) 7 ]; the molar ratio of the β-cyclodextrin, triphenylphosphine and iodine simple substance is 1: 1.3: 1.5; the anhydrous N, N-dimethylformamide is added with 15 grams of dry Beta-cyclodextrin meter; the molar number of sodium methylate in the sodium methylate solution is 1.8 times of the dry beta-cyclodextrin molar number;
[0062] b. Dissolve the hepta(6-iodo-6-deoxy)-β-cyclodextrin and sodium azid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com