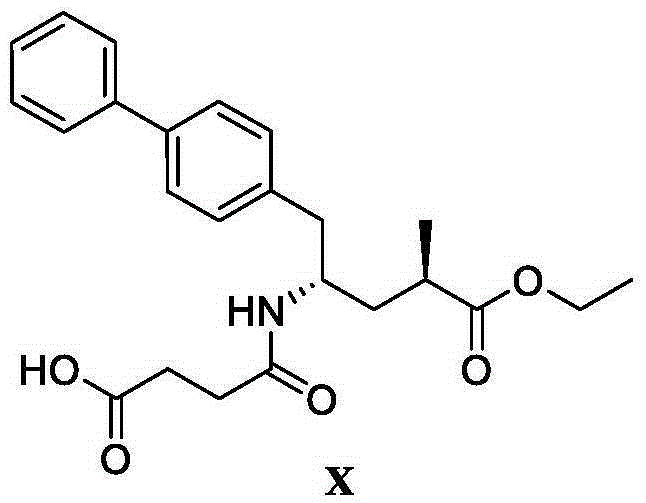
Preparation method of (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol
A technology of tert-butoxycarbonylamino and aminobiphenylpropanol, which is applied in the field of preparation of Sacubitril intermediates, can solve the problems of high requirements for reaction operations, heavy environmental pollution, and inability to solve the synthesis of target compounds well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086]Embodiment 1: (R)-N-acetylbiphenylalanine ethyl ester
[0087] Step (1): (4Z)-4-Biphenylmethylene-2-methyl-1,3-oxazol-5-one
[0088] Under nitrogen protection, N-acetylglycine (0.45mol, 52.7g), anhydrous sodium acetate (0.5mol, 41.0g), and acetic anhydride (1mol, 102.1g) were successively put into a 1L three-necked flask, and mechanically stirred for 30min at room temperature , slowly add biphenyl formaldehyde (0.5mol, 91.1g), heat up to 90°C, keep the temperature of the reaction system at 85-95°C, follow TLC until the reaction of the raw material point is complete. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours to crystallize. The material was extracted and dried in vacuo to obtain 102.5 g of a yellow solid product (4Z)-4-biphenylmethylene-2-methyl-1,3-oxazol-5-one, with a yield of 86.5%.
[0089] Step (2): (Z)-Ethyl 2-acetamido-3-biphenylacrylate
[0090] At room temperature, add the produc...
Embodiment 2
[0094] Embodiment 2: (R)-N-benzoyl biphenylalanine methyl ester
[0095] Step (1): (4Z)-4-Biphenylmethylene-2-phenyl-1,3-oxazol-5-one
[0096] Under nitrogen protection, N-benzoylglycine (0.5mol, 89.6g), anhydrous potassium acetate (0.6mol, 58.9g), acetic anhydride (2mol, 204.2g) were successively dropped into a 1L three-necked flask, and mechanically Stir for 30 minutes, add biphenyl formaldehyde (0.5mol, 91.1g) slowly, heat up to 95°C, keep the temperature of the reaction system at 90-100°C, follow TLC until the reaction of the raw material point is complete. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours to crystallize. The material was extracted and dried in vacuo to obtain 150.3 g of a yellow solid product (4Z)-4-biphenylmethylene-2-phenyl-1,3-oxazol-5-one, with a yield of 92.4%.
[0097] Step (2): (Z)-Methyl 2-benzamido-3-biphenylacrylate
[0098] At room temperature, add the product obtained...
Embodiment 3
[0102] Embodiment 3: (R)-N-tert-butoxycarbonyl biphenylalanine
[0103] Step (1): (4Z)-4-Biphenylmethylene-2-tert-butoxy-1,3-oxazol-5-one
[0104] Under the protection of nitrogen, N-tert-butoxycarbonylglycine (0.6mol, 105.1g), anhydrous sodium acetate (0.55mol, 45.1g), and acetic anhydride (1.5mol, 153.1g) were successively added to a 1L three-necked flask at room temperature. Stir mechanically at low temperature for 30 minutes, slowly add biphenyl formaldehyde (0.5mol, 91.1g), heat up to 90°C, keep the temperature of the reaction system at 85-95°C, follow TLC until the reaction of the raw material point is complete. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours to crystallize. The material was extracted and dried in vacuo to obtain 126.4 g of a yellow solid product (4Z)-4-biphenylmethylene-2-tert-butoxy-1,3-oxazol-5-one, with a yield of 78.7%.
[0105] Step (2): (Z)-2-tert-butoxycarbonylamino-3-b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com