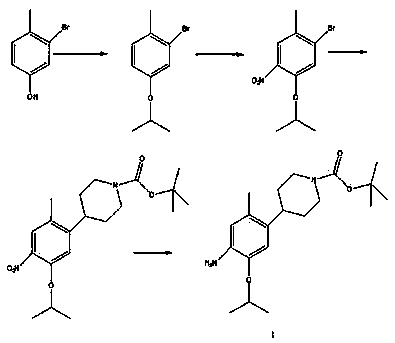
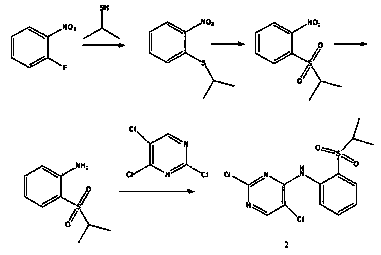
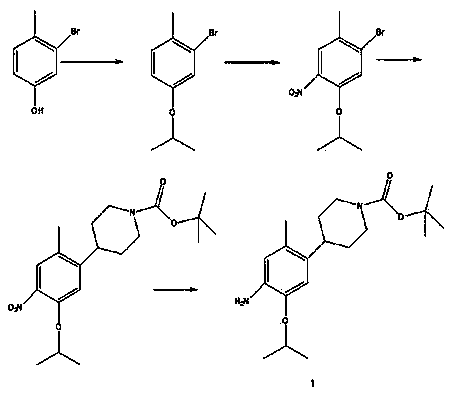
Method for preparing ceritinib
A technology of ceritinib and intermediates, which is applied in the field of preparation of ceritinib, can solve the problems of high cost, low yield, difficult industrialization and the like, and achieves the effects of low cost, simple operation and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 2-isopropylmercaptonitrobenzene
[0026] O-Fluoronitrobenzene (10g, 10mmol), add 100ml DMF, add 2-mercaptoisopropanol (5.4g, 70mmol), 25g potassium carbonate, warm to 100 degrees and react for 16h, cool to room temperature, add 500ml water, ethyl acetate (100ml*3) Extract, combine the organic layers, wash with water, wash with saturated brine, dry with anhydrous sodium sulfate, and concentrate to obtain 13.3g of light buttery substance, with a yield greater than 100%, without purification.
Embodiment 2
[0027] Example 2: Preparation of 2-(isopropylsulfonyl)nitrobenzene
[0028] O-Isopropyl mercaptonitrobenzene (13g, 65.97mmol), 120ml of dichloromethane, add m-chloroperoxybenzoic acid (25.67g, 149.42mmol) in batches at room temperature, stir at room temperature for 16h, filter, and concentrate to dryness to obtain a yellowish brown Solid 12g, yield 80%, not purified.
Embodiment 3
[0029] Example 3: Preparation of 2-(isopropylsulfonyl)aniline
[0030] 2-(isopropylsulfonyl)nitrobenzene (2g, 8.73mmol), add 30ml methanol, 0.2g 10% palladium on carbon, hydrogenate at atmospheric pressure for 6h, filter with suction, concentrate and spin off methanol to obtain 1.5g of light butter , The yield is 88%, without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com