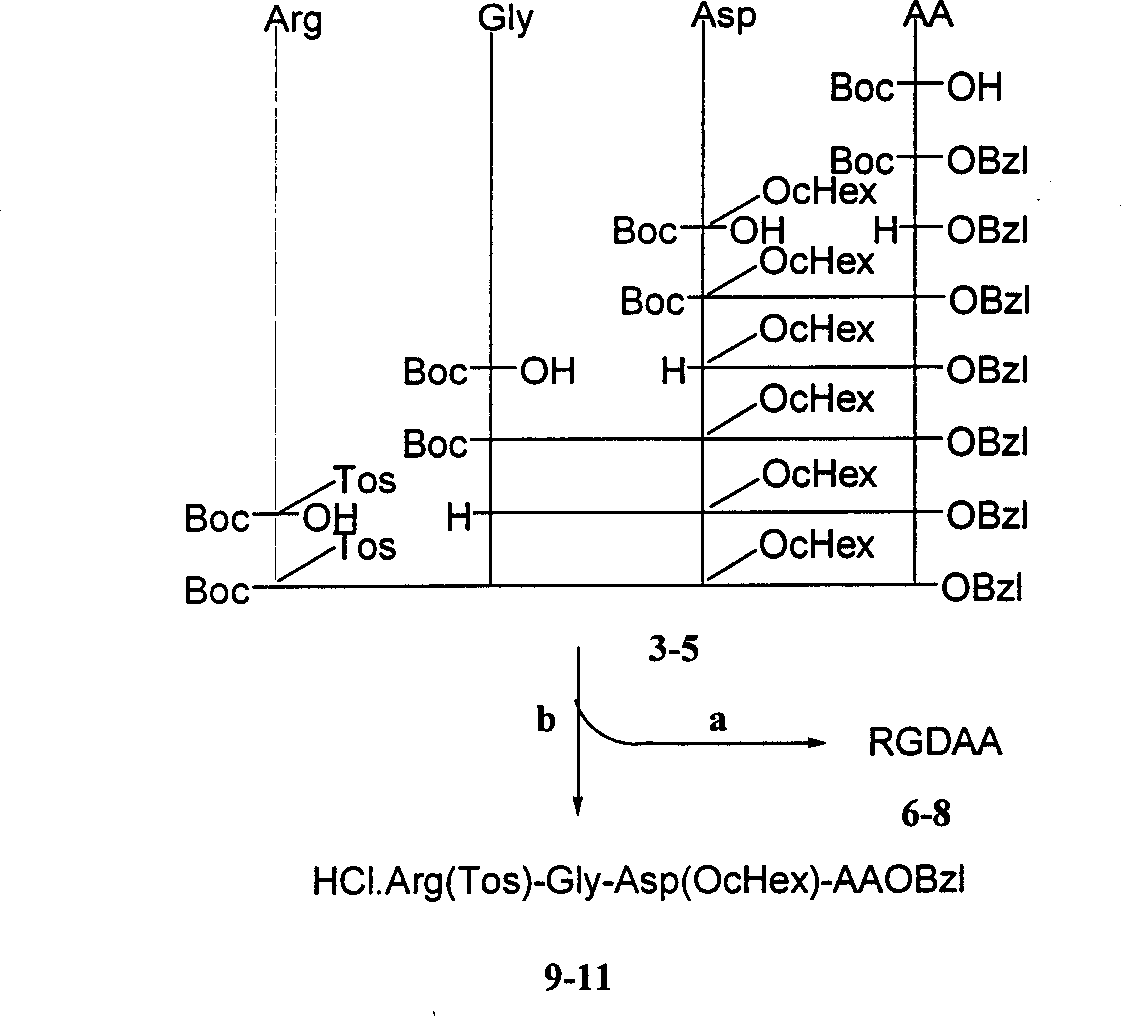
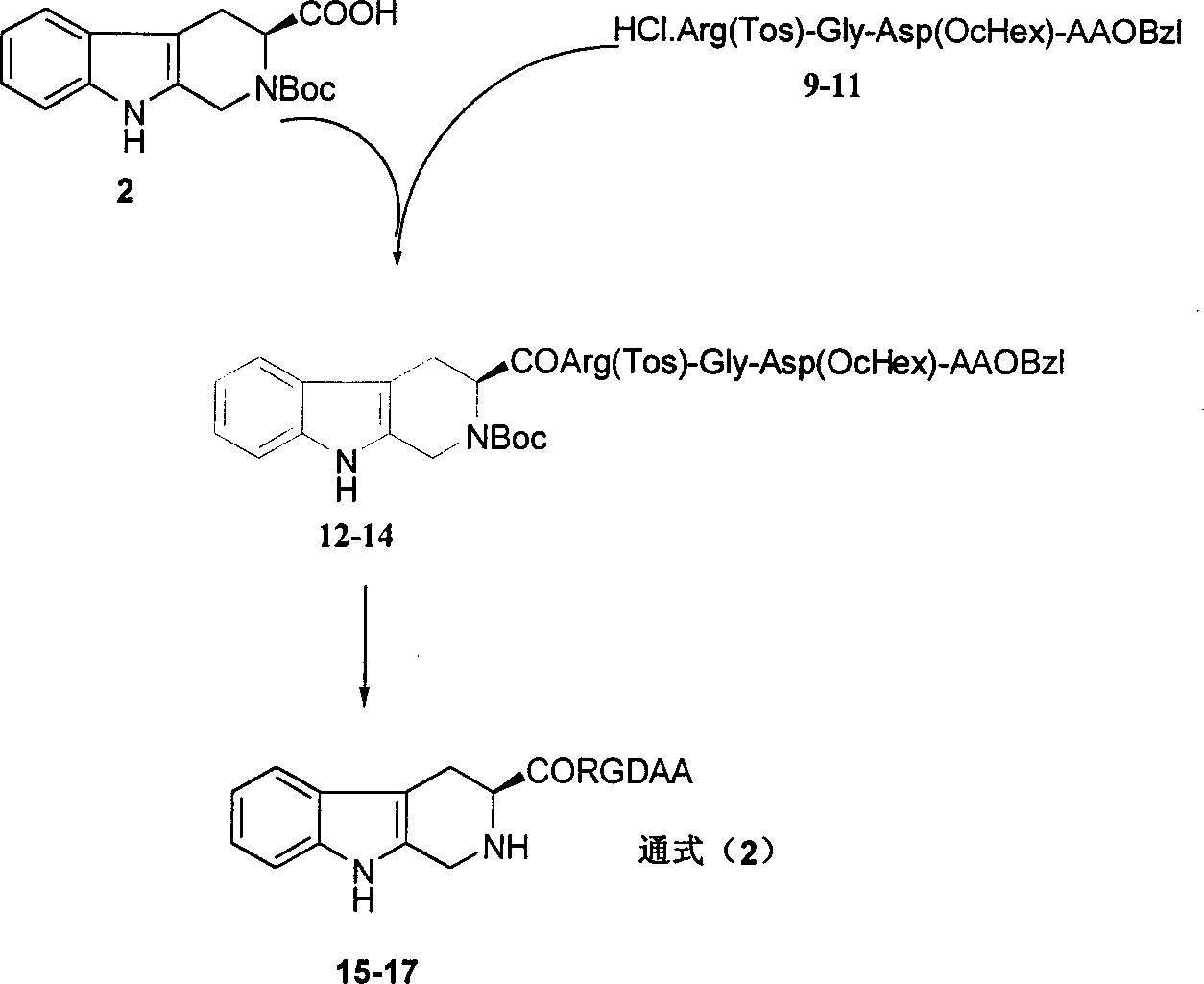
Beta-tetrahydro carboline carboxylic acid, its RGD conjugate, their synthesis and medical application
A tetrahydrocarboline carboxylic acid and medical technology, which is applied in the field of β-tetrahydrocarboline carboxylic acid and its RGD conjugates, can solve the problems of poor water solubility and inconvenient biological activity determination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Embodiment 2
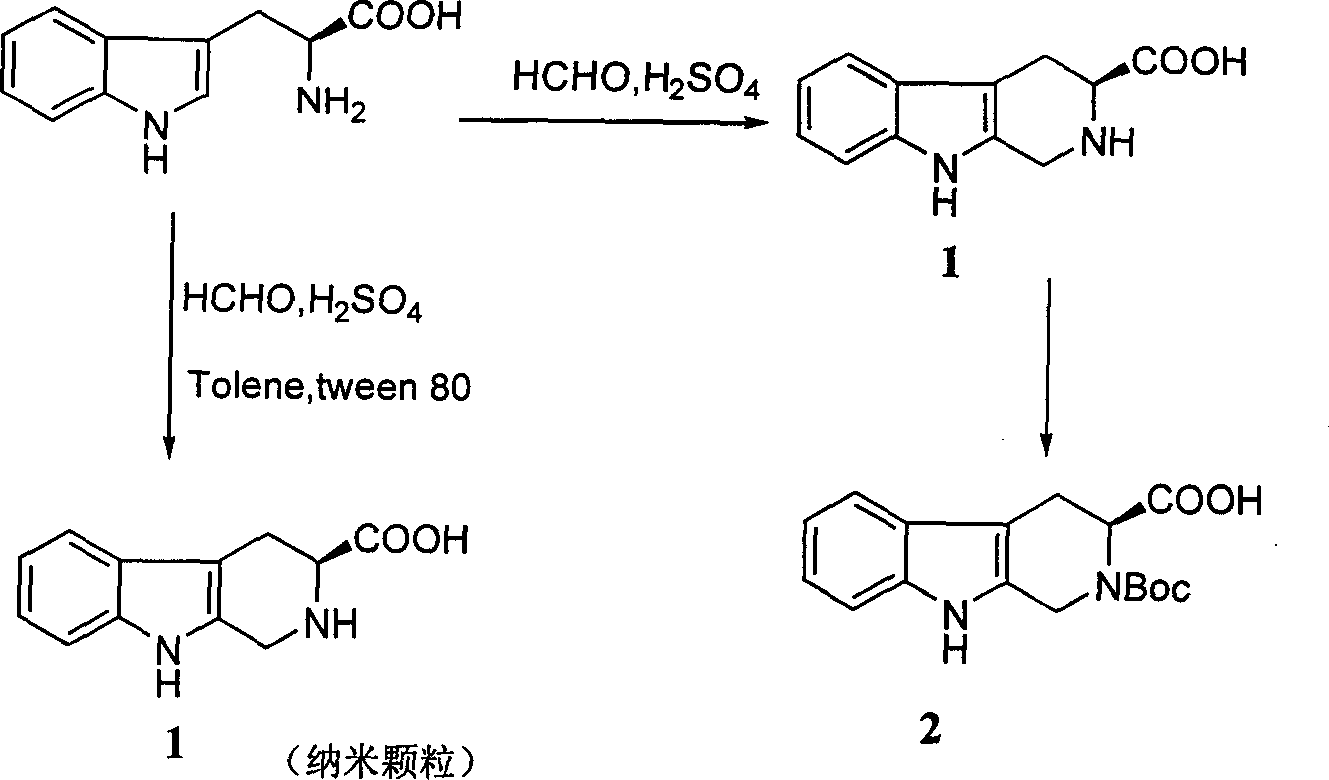
[0015] 25ml of 1N H2SO4, add 5g (24.5mmol) tryptophan while stirring, add 75ml of water, 4ml (45.6mmol) 38% formaldehyde solution, the reaction solution becomes clear, and a large amount of solids are precipitated in about 5Min. After reacting for 4 hours, add dropwise 8ml of concentrated ammonia water, adjust the pH to nearly neutral, let stand overnight, filter with suction, wash the filter cake with a small amount of cold water, drain, and then dry to obtain 5.1g of solid (1) (light yellow powder, yield 96%) MP: 280 -282 C; EI-MS: 217 (M+1); H-NMR (DMSO): δ10.99 (1H, s), 7.44 (1H, d, 7.5), 7.33 (1H, t, 8.0), 7.08 (1H, t, 8.0), 6.99 (1H, t, 7.5), 4.22 (1H, d, 4.8), 3.69 (1H, dd, 10.5, 5.0), 3.14 (1H, dd, 10.5, 2.4), 2.83 ( 1H, ddd, 10.5, 5.0, 2.4) Example 2: Preparation of N-Boc-β-tetrahydrocarbolinecarboxylic acid (2)
Embodiment 3
[0016] Suspend 1.1g (5mmol) of compound 87 in 15ml of DMF, add 1.4ml of triethylamine, stir under ice bath until the suspended particles in the solution are uniform in size, add 1.1g (7.7mmol) Boc-N3 dropwise within 30min, and rise to to 40°C, after 3 days, add 5ml of 20% citric acid aqueous solution, extract with ethyl acetate, organic layer is dried with MgSO4, filtered, the filtrate is distilled to dryness under reduced pressure, and the residue is recrystallized with CHCl3 to obtain the title compound 0.9g (56% )mp165-170 TOF-MS: 317[M+1] + , 339[M+Na] + , 355[M+K] 1 H-NMR (DMSO-d 6 ).1.46(s, 9H, -Boc), 3.25~3.34(m, 2H, 4-H), 4.29~4.75(m, 2H, 1-H), 5.02~5.14(m, 1H, 3-H) , 6.92~7.41 (m, 4H, Ar-H (5, 6, 7, 8)), 10.87 (s, 1H, 9-H), 12.77 (s, 1H, -COOH) Example 3: β-four Preparation of Hydrocarboline Carboxylic Acid Nanoparticles (1)
[0017] Add 0.05ML concentrated H2SO4 to 10ML deionized water, add 1G L-tryptophan while stirring, then add 20ML toluene, 0.02ML TWEEN80, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com