Pentazane derivative intermediate, its preparation and use
A derivative, pyrrolidine technology, applied in the field of chemical synthesis, can solve the problems of difficult removal of impurities, high cost, and difficulty in product purification, and achieve the effects of avoiding side reactions and simple preparation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
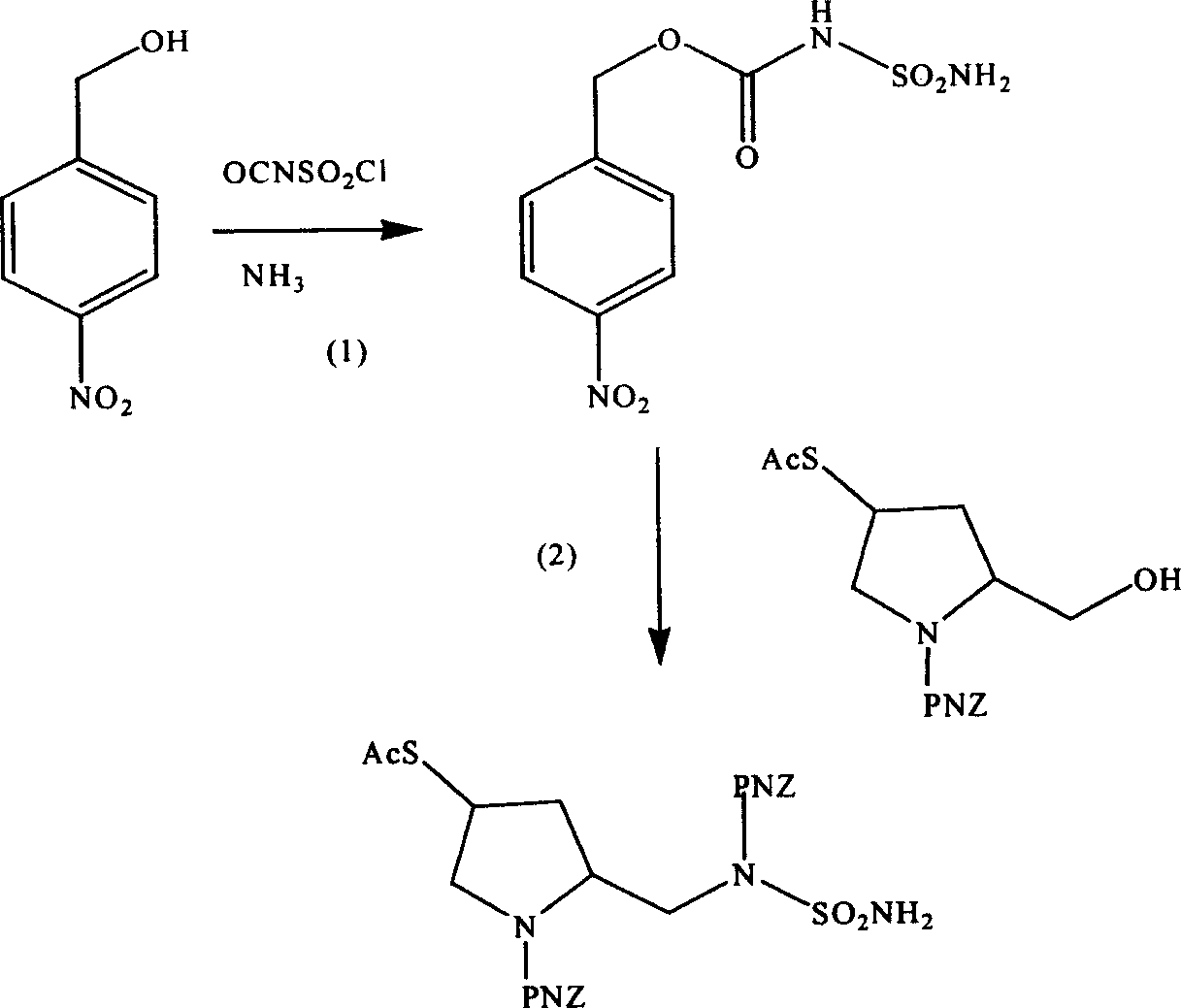
[0031] The preparation of embodiment 1 pyrrolidine derivatives
[0032] Step 1N-Preparation of p-nitrobenzyloxycarbonylsulfonamide
[0033] Cool 38.285g (250mmol) of p-nitrobenzyl alcohol tetrahydrofuran solution to -40°C, add dropwise 21.75ml (250mmol) chlorosulfonyl isocyanate therein, continue stirring for 30 minutes after the dropwise addition, and then pass ammonia gas for 30 minutes Minutes later, the reaction mixture was acidified with 1 N hydrochloric acid, and the resulting precipitate was dissolved in ethyl acetate, washed with water and brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure and recrystallized in ethyl acetate-hexane to obtain a white 55 g of flaky crystals (80% yield), melting point: 160-162°C.
[0034] ESI-MS: 298[M+Na] + , 314[M+K] +
[0035] 1 HNMR (600MHz, DMSO-d 6 )δ5.29(s, 2H, -O-CH 2 -Ar), 7.54 (br, 2H, -SO 2 NH 2 ), 7.64 (A2 B 2 , 2H, J=8.7Hz, -ArNO 2 meta), 8.26 (A 2 B 2 , 2H, J=8.7Hz, -ArNO 2 ortho), ...
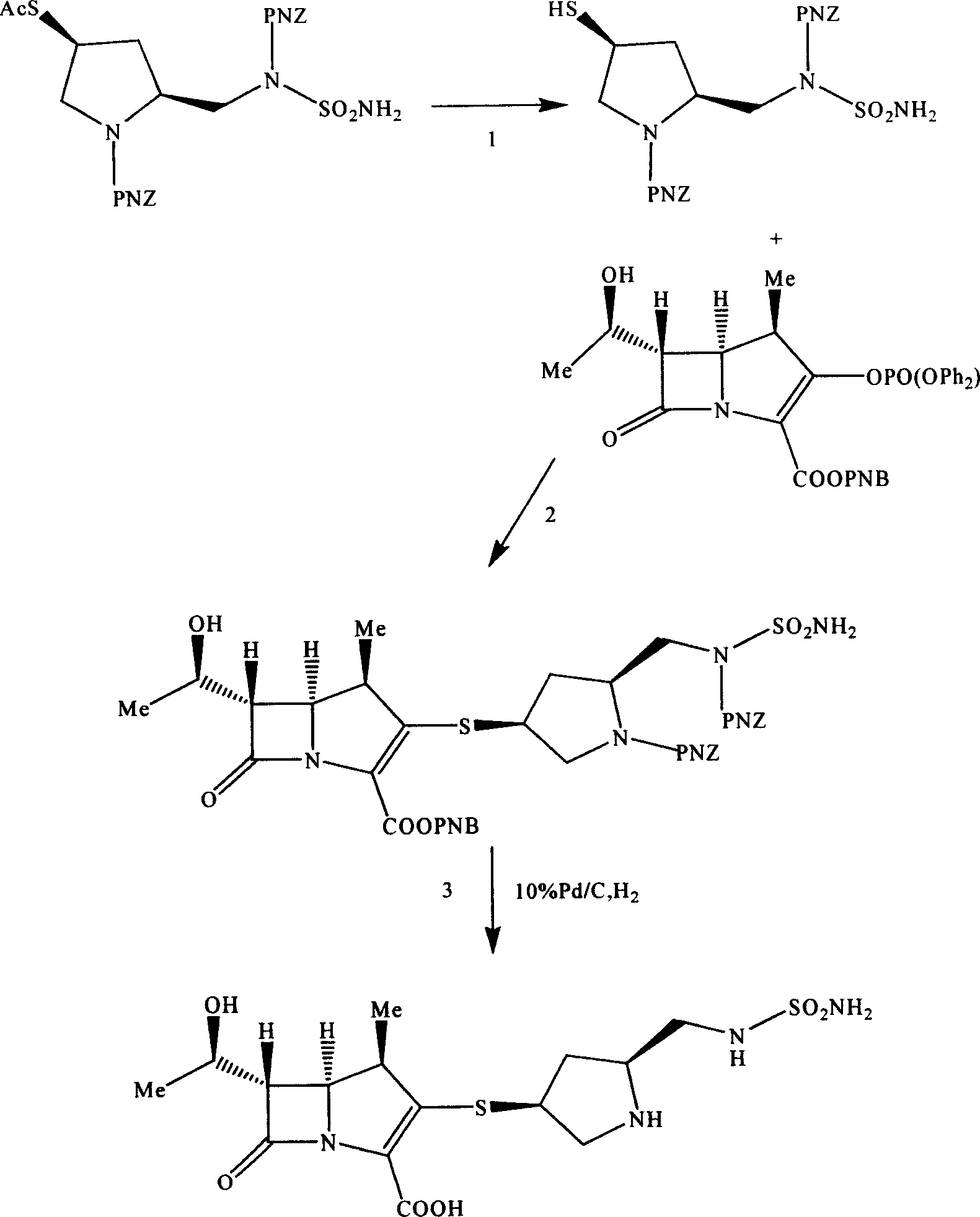
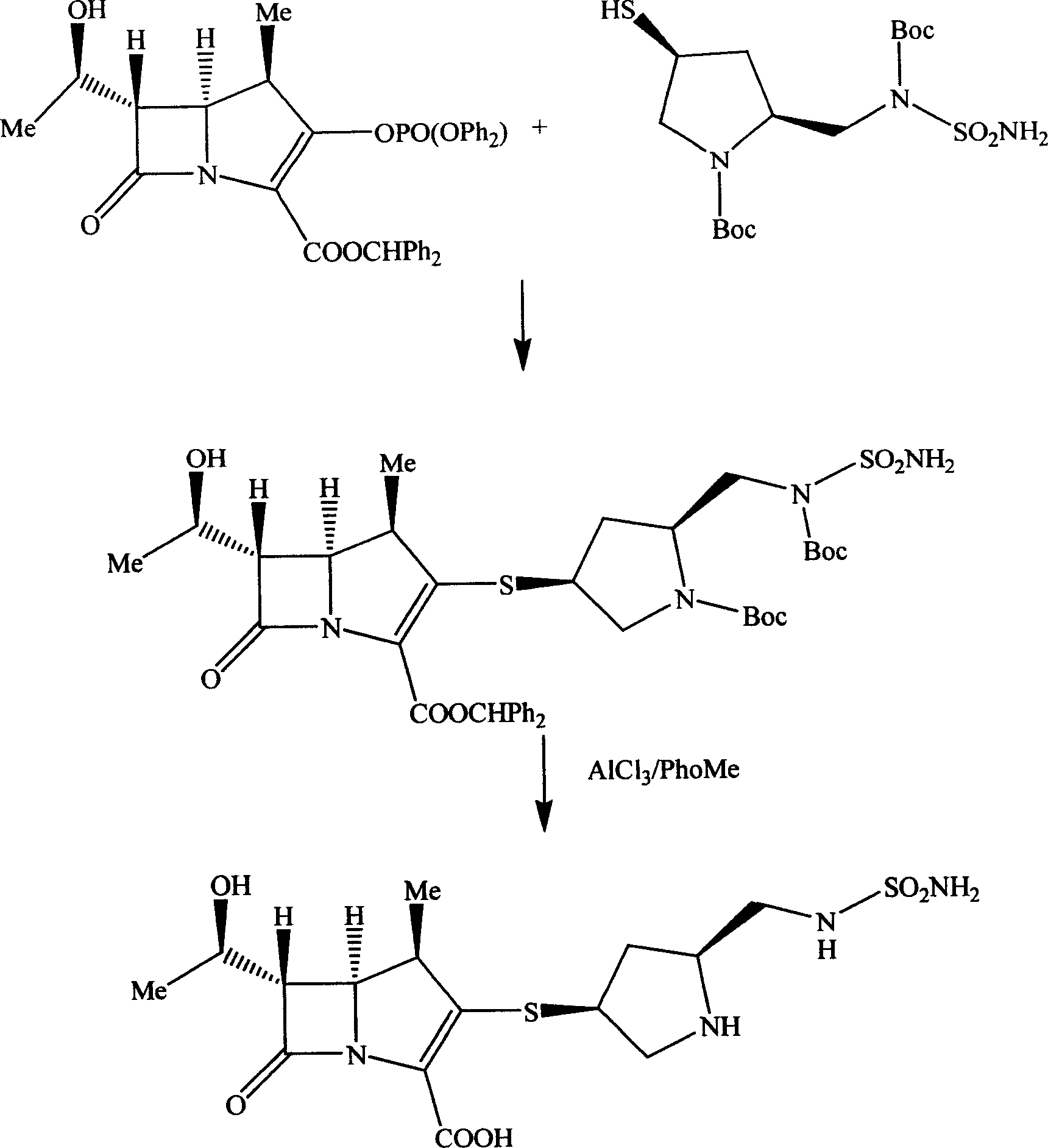
Embodiment 2
[0049] The synthesis of embodiment 2 (3S, 5S)-pyrrolidinylthio carbapenem derivatives
[0050] The preparation of step 1 mercaptopyrrolidine derivative
[0051] (2S, 4S)-1-p-nitrobenzyloxycarbonyl-2-(N-p-nitrobenzyloxycarbonyl-N-sulfamoylamino)methyl-4-acetylthiopyrrolidine 50 g (81.8 mg mol) was dissolved in 200 milliliters of tetrahydrofuran, and 20 milliliters of aqueous solution of 6 grams of lithium hydroxide was added dropwise under an ice bath. After the addition was completed, stirring was continued for 120 minutes, acidified with 6 equivalents of hydrochloric acid, and a solid viscous substance was precipitated, which was washed with ethyl acetate Add ethanol after dissolving, freeze and precipitate 32 grams of light yellow amorphous solid powder, yield 68.8%.
[0052] ESI-MS: 592.1[M+Na], 608.1[M+K] +
[0053] 13 C NMR (600MHz, CD 3 COCD 3 ) δ34.3 (pyrrole ring C-3), 39.0 (pyrrole ring C-4),
[0054] 50.2(-CH 2 NSO 2 -), 55.3 (pyrrole ring C-5), 57.1 (pyrrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com