Chirality covalent organic framework and synthesis method and application thereof
A covalent organic framework and synthesis method technology, applied in the field of chiral covalent organic framework materials and their synthesis, to achieve good catalytic activity and cycle stability, large specific surface area, and extended application effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) The synthesis method of chiral precursor 1 is as follows:
[0035]
[0036] Synthesis of compound 3:
[0037] Take a one-necked bottle, add N-tert-butoxycarbonyl-L-proline (5.17g, 24.0mmol), anhydrous dichloromethane (60mL), stir to dissolve and cool to 0°C. Ethyl chloroformate (2.28mL, 24.0mmol) and anhydrous triethylamine (6.65mL, 48.0mmol) were slowly added dropwise, and then 3,6-dibromo-1,2-phenylenediamine (5.32g, 20.0 mmol). The reaction system was allowed to warm to room temperature, and the reaction was stirred at room temperature for 36h. After the reaction, 100 mL of water was added to the reaction system, extracted with dichloromethane, the organic phase was washed with saturated brine, dried with anhydrous sodium sulfate, and the solvent was evaporated to obtain a brown oil. Glacial acetic acid (25 mL) was added to the brown oil, and the reaction was stirred at 65° C. for 12 h. After the reaction, cool to room temperature, add saturated sodium car...
Embodiment 2
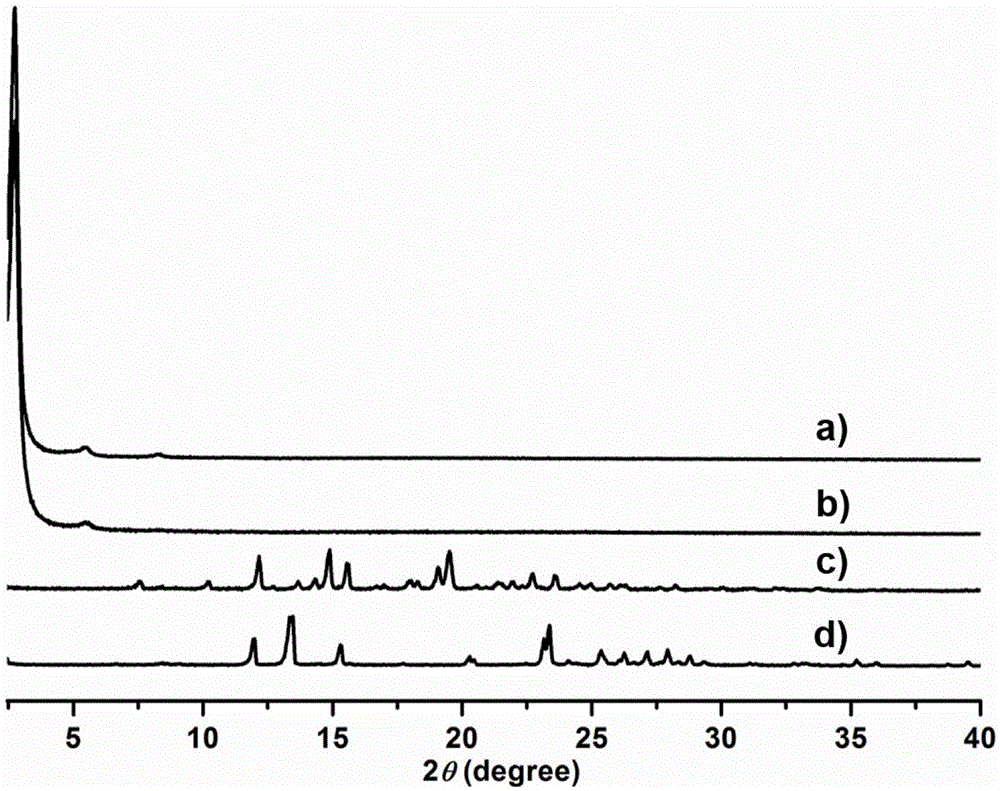
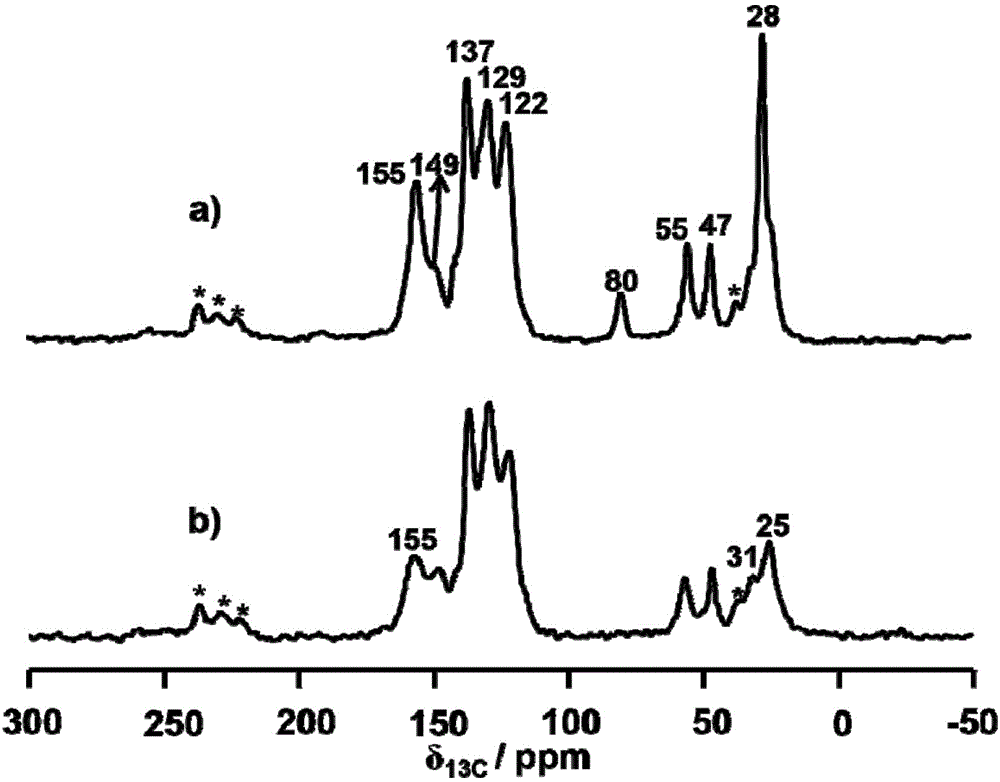
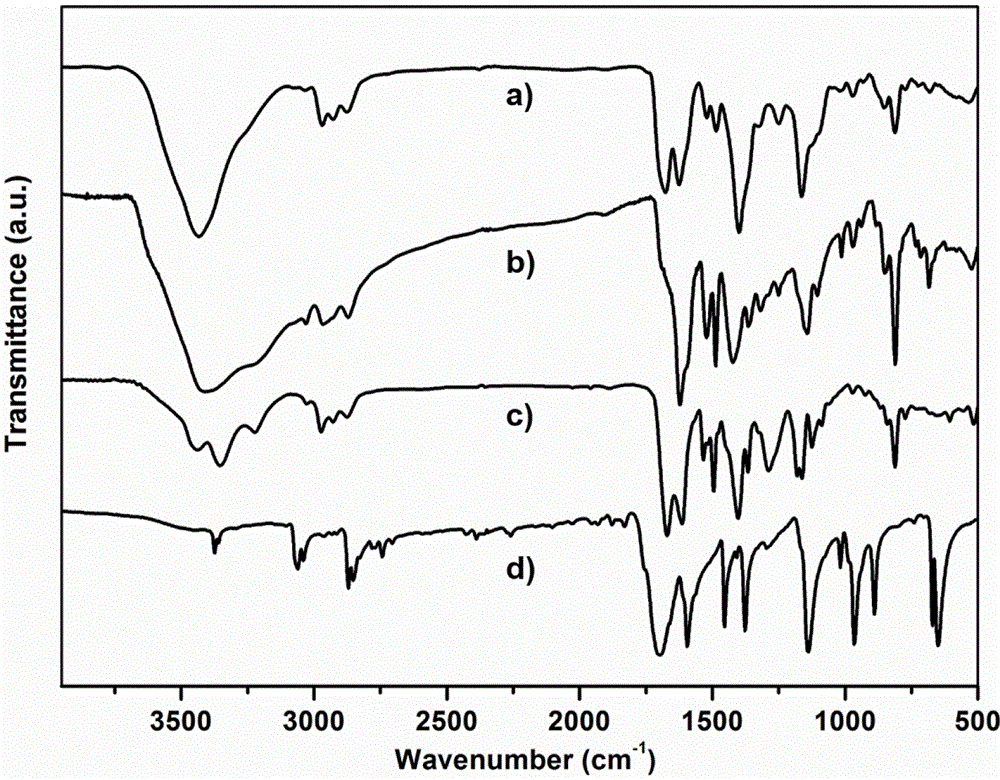
[0061] Add 8.1 mg of trimesaldehyde and 35.2 mg of chiral precursor 1 into the pressure-resistant tube. Then add 0.2mL absolute ethanol and 0.8mL mesitylene, shake well and add 0.10mL 3mol / L acetic acid solution. After sealing the pressure tube with a rubber stopper, replace it with argon three times, then quickly remove the rubber stopper, and seal the pressure tube with a polytetrafluoroethylene stopper. It was placed in an oven and reacted at 100° C. for three days. After the reaction, a solid was produced at the bottom of the pressure-resistant tube, and the solid was transferred to a centrifuge tube, washed with acetone and tetrahydrofuran for 3 times, respectively. Dry the solid at 100°C to obtain CCOF-LZU72-Boc.
[0062] CCOF-LZU72-Boc (15.0 mg) was placed in a pressure-resistant tube, replaced with argon three times, and then sealed with a polytetrafluoroethylene stopper. Place it in an oven preheated to 235°C and heat for 25 minutes to obtain CCOF-LZU72.
Embodiment 3
[0064] Add 16.2 mg of trimesaldehyde and 70.4 mg of chiral precursor 1 into the pressure tube. Then add 0.2mL tetrahydrofuran and 1.8mL mesitylene, shake well and add 0.4mL 3mol / L acetic acid solution. After sealing the pressure tube with a rubber stopper, replace it with argon three times, then quickly remove the rubber stopper, and seal the pressure tube with a polytetrafluoroethylene stopper. It was placed in an oven and reacted at 80° C. for three days. After the reaction, a solid was produced at the bottom of the pressure-resistant tube, and the solid was transferred to a centrifuge tube, washed with acetone and tetrahydrofuran for 3 times, respectively. Dry the solid at 100°C to obtain CCOF-LZU72-Boc.
[0065] CCOF-LZU72-Boc (15.0 mg) was placed in a pressure-resistant tube, replaced with argon three times, and then sealed with a polytetrafluoroethylene stopper. Place it in an oven preheated to 245°C, and heat it for 15 minutes to obtain CCOF-LZU72.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com