Method of synthesizing amide with methyl aromatics and amine in water phase
A technology for synthesizing methyl aromatic hydrocarbons and amides, applied in chemical instruments and methods, formation/introduction of amide groups, preparation of carboxylic acid amides, etc., can solve problems such as applicability limitations, and achieve cost reduction and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018]
[0019] In a clean 10 mL sealed tube add toluene (20 eq) and butylamine (0.24 mmol), TBHP (70% water) 6 eq as oxidant, 30 mol% TBAI and 15 mol% FeCl 3 As catalyst, 100 mg of 4A molecular sieves and 1.5 mL of water. at 60 o C for 24 hours, TLC plate detection, after the reaction, the reaction solution was cooled to room temperature, the reaction solution was extracted with ethyl acetate (3 × 10 mL), the organic phase was combined and washed with anhydrous Na 2 SO 4 After drying, the organic layer was concentrated and subjected to column chromatography to obtain pure N-butylbenzamide as a yellow liquid with a yield of 73%.
[0020] 1 H NMR (400 MHz, CDCl 3 ): δ 7.75 (d, J = 7.7 Hz, 2H), 7.48–7.37(m, 3H), 6.36 (bs, 1H), 3.45–3.40 (m, 2H), 1.64–1.53 (m, 2H), 1.43 –1.36 (m, 2H), 0.93 (t, J = 7.2 Hz, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 167.5, 134.9, 131.2, 128.4, 126.8, 39.8, 31.7, 20.1, 13.7.
example 2
[0022]
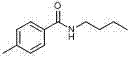
[0023] In a clean 10 mL sealed tube add p-xylene (20 eq) and butylamine (0.24 mmol), TBHP (70% water) 6 eq as oxidant, 30 mol% TBAI and 15 mol% FeCl 3 As catalyst, 100 mg of 4A molecular sieves and 1.5 mL of water. at 60 o C for 24 hours, TLC plate detection, after the reaction, the reaction solution was cooled to room temperature, the reaction solution was extracted with ethyl acetate (3 × 10 mL), the organic phase was combined and washed with anhydrous Na 2 SO 4 After drying, the organic layer was concentrated and subjected to column chromatography to obtain pure N-butyl-4-methylbenzamide as a yellow solid with a yield of 78%.
[0024] 1 H NMR (400 MHz, CDCl 3 ): δ 7.65 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 7.9 Hz, 2H), 6.30 (bs, 1H), 3.44–3.39 (m, 2H), 2.37 (s, 3H), 1.61–1.54 (m, 2H), 1.43–1.34 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H); 13 C NMR (100 MHz, CDCl 3 ): δ 167.4, 141.5, 132.0, 129.1, 126.8, 39.7, 31.7, 21.3, 20.1, 13.7.
example 3
[0026]
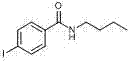
[0027] In a clean 10 mL sealed tube add 4-methylanisole (20 eq) and butylamine (0.24 mmol), TBHP (70% water) 6 eq as oxidant, 30 mol% TBAI and 15 mol% FeCl 3 As catalyst, 100 mg of 4A molecular sieves and 1.5 mL of water. at 60 o C for 24 hours, TLC plate detection, after the reaction, the reaction solution was cooled to room temperature, the reaction solution was extracted with ethyl acetate (3 × 10 mL), the organic phase was combined and washed with anhydrous Na 2 SO 4 After drying, the organic layer was concentrated and subjected to column chromatography to obtain pure benzene N-butyl-4-methoxybenzamide as a yellow liquid with a yield of 82%.
[0028] 1 H NMR (400 MHz, CDCl 3 ): δ 7.72 (d, J = 7.7 Hz, 2H), 6.87 (d, J = 7.8 Hz, 2H), 6.31 (bs, 1H), 3.81 (s, 3H), 3.53–3.29 (m, 2H), 1.58–1.52 (m, 2H), 1.40–1.34 (m, 2H), 0.92 (t, J = 6.7 Hz, 3H) 13 C NMR (100 MHz, CDCl 3 ): δ 167.0, 161.9, 128.6, 127.0, 113.5, 55.3, 39.7, 31.7, 20.1, 13.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com