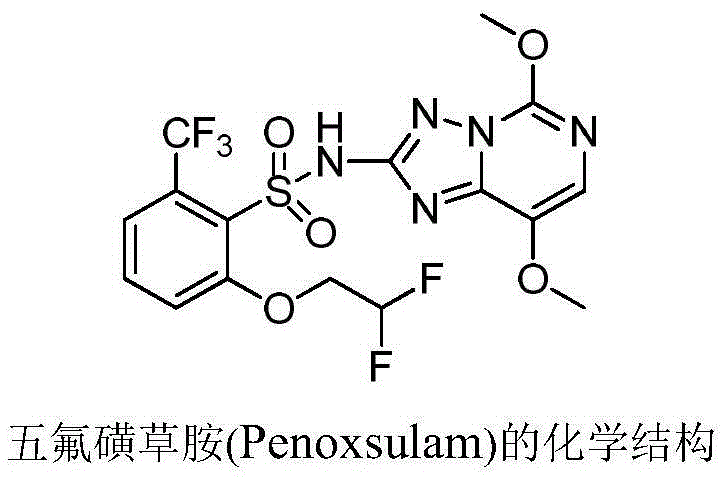
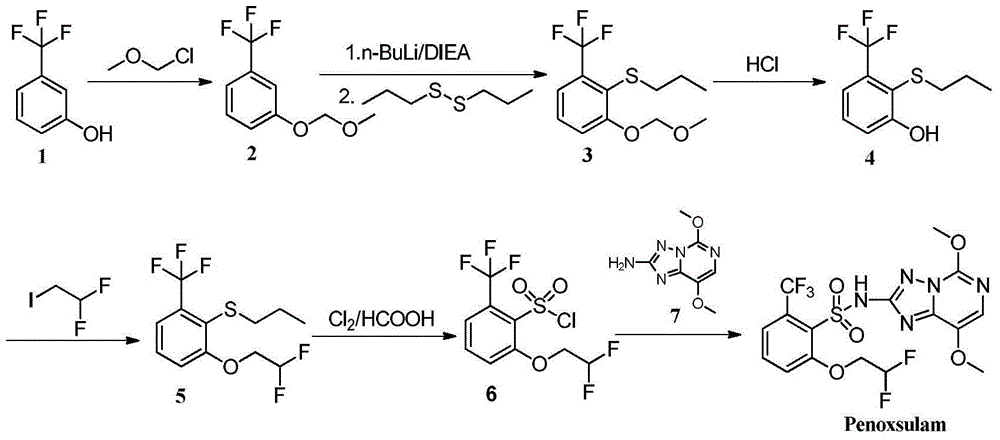
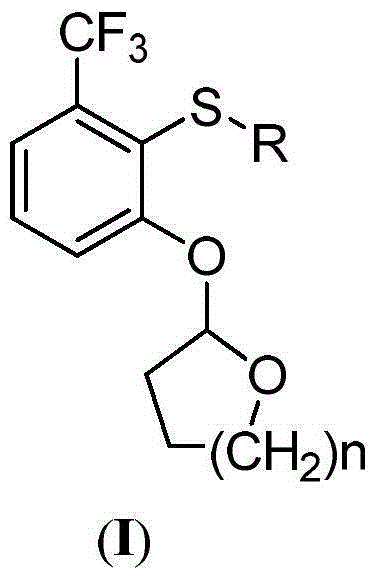
2-phenoxyl tetrahydrofuran (tetrahydropyrane) derivatives and application thereof in synthesis of penoxsulam
A technology of phenoxytetrahydrofuran and tetrahydrofuran, which is applied in the direction of organic chemistry, can solve the problems of difficult preparation, high price, and low yield, and achieves cheap and easy-to-obtain synthetic raw materials, less by-products, and improved product quality. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 2-(2-(methylthio)-3-(trifluoromethyl)phenoxy)tetrahydrofuran (I-1)
[0027] At room temperature, slowly drop 10 g m-trifluoromethylphenol (61.69 mmol) into 1,4-dioxane (30 mL) dissolved in 2,3-dihydrofuran (14 mL) and anhydrous hydrogen chloride (0.15 g) , stirred overnight, the reaction solution was evaporated to dryness, ethyl acetate (30mL) and saturated sodium bicarbonate solution (30mL) were added to the obtained raffinate, the organic layer was dried over anhydrous sodium sulfate for 2h, then filtered and evaporated to dryness, reduced pressure Distillation gave 13.31 g of the compound 2-(3-(trifluoromethyl)phenoxy)tetrahydrofuran, with a yield of 93%. 1 H NMR (400MH z, CDCl 3 )δ7.41-7.22(m, 4H, Ar-H), 5.49(t, J=3.2Hz, 1H, furan ring hydrogen), 3.82-3.74(m, 2H, furan ring hydrogen), 2.37-2.30(m ,1H,furan ring hydrogen),2.41-2.32(m,1H,furan ring hydrogen),1.92-1.80(m,2H,furan ring hydrogen);ESI-MS:233[M+H + ].
[0028] N 2 Under protection, 2-(3-(tr...
Embodiment 2
[0029]Example 2: 2-(2-(ethylthio)-3-(trifluoromethyl)phenoxy)tetrahydrofuran (I-2)
[0030] With 2-(3-(trifluoromethyl)phenoxy)tetrahydrofuran as raw material, with reference to the method of Example 1, under the action of n-butyllithium, diethyl disulfide is used to react (other raw materials are the same), The target product I-2 was obtained with a yield of 89%, 1 H NMR (400MHz, CDCl 3 )δ7.20(d, 1H, J=8.0Hz, Ar-H), 7.13-7.02(m, 2H, Ar-H), 5.58(t, J=3.2Hz, 1H, furan ring hydrogen), 3.84- 3.72(m, 1H, furan ring hydrogen), 3.64-3.51(m, 1H, furan ring hydrogen), 2.85(q, 2H, CH 2 ,J=6.4Hz),2.42-2.36(m,1H,furan ring hydrogen),2.25-2.17(m,1H,furan ring hydrogen),1.94-1.84(m,2H,furan ring hydrogen),1.30(t, 3H,CH 3 , J=6.4Hz); ESI-MS: 293[M+H + ].
Embodiment 3
[0031] Example 3: 2-(2-(propylthio)-3-(trifluoromethyl)phenoxy)tetrahydrofuran (I-3)
[0032] With 2-(3-(trifluoromethyl)phenoxy)tetrahydrofuran as raw material, with reference to the method of Example 1, under the action of n-butyllithium, dipropyl disulfide is used to react (other raw materials are the same), The target product I-3 was obtained with a yield of 91%, 1 H NMR (400MHz, CDCl 3 )δ7.20(d, 1H, J=8.0Hz, Ar-H), 7.13-7.02(m, 2H, Ar-H), 5.56(t, J=3.2Hz, 1H, furan ring hydrogen), 3.85- 3.73(m, 1H, furan ring hydrogen), 3.63-3.51(m, 1H, furan ring hydrogen), 2.83(t, 2H, CH 2 ,J=6.4Hz), 1.63(m,2H,CH 2 ),1.01(t,3H,CH 3 , J=6.4Hz), 2.41-2.32 (m, 1H, furan ring hydrogen), 2.22-2.16 (m, 1H, furan ring hydrogen), 1.94-1.83 (m, 2H, furan ring hydrogen); ESI-MS: 307[M+H + ].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com