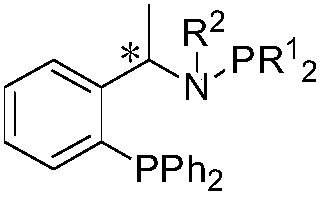
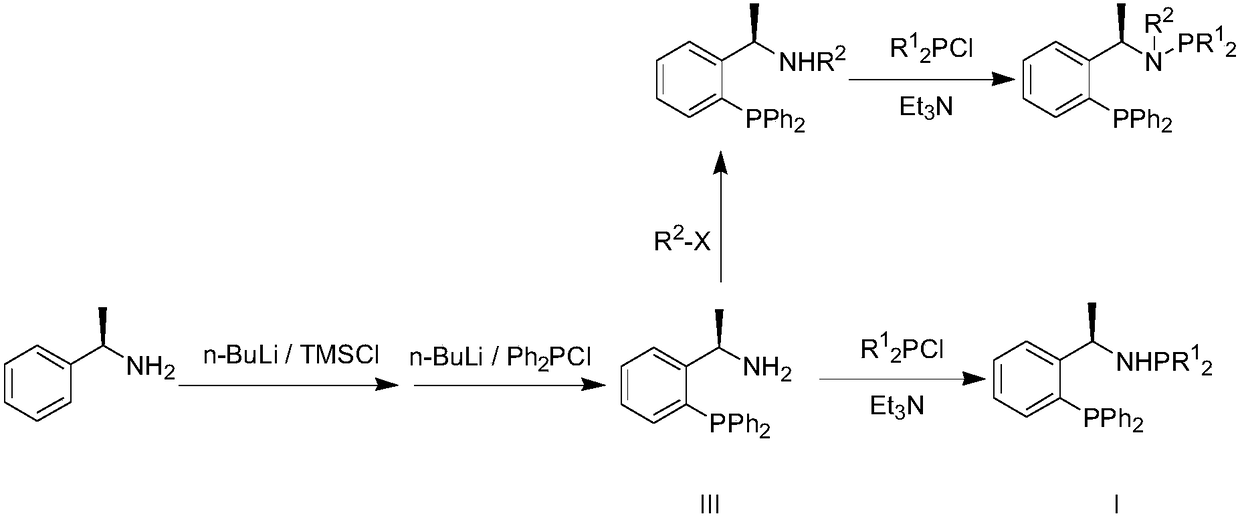
Method for synthesizing chiral amine compound through catalyzing asymmetric hydrogenated imine by using iridium
A technology of hydrogenated imines and synthetic methods, applied in asymmetric synthesis, preparation of organic compounds, organic chemical methods, etc., can solve problems such as high equipment requirements, difficult ligand synthesis, etc., and achieve high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1 Preparation of Ligand I a
[0036] At minus 20 degrees, add n-butyllithium (0.1mol) dropwise to the ether solution of 12.118g of α-phenylethylamine, and then stir for 30min, then add trimethylchlorosilane (0.1mol), and stir for 30min Then add n-butyllithium (0.13mol) dropwise, and stir for 1h; then add diphenylphosphorous chloride (0.11mol) dropwise therein, stir for 2h after adding, rise to room temperature and stir for 4h, and then use 10% HCl (aq) Quench the reaction, separate the layers, spin the organic layer to dryness, and recrystallize with n-hexane to obtain a white solid (abbreviated as DPPNH 2 ), yield 48%.
[0037] To 3.0535g DPPNH 2 Add 2.5050 g of diphenylphosphine chloride to the toluene solution of (1 equiv) under the condition of 5 ml of triethylamine. After the reaction is finished, the salt generated by the reaction is filtered off, the organic phase is spin-dried, and recrystallized with n-hexane to obtain the ligand I a , yield 95%.
[0038] ...
Embodiment 2
[0057] The hydrogenation reaction was carried out in a 200ml autoclave. First the reactor was replaced with nitrogen three times, then 52g of imine (generated by 2-methyl-6-ethylaniline and methoxyacetone) was injected into the reactor, and then 0.5ml of in-situ coordinated catalyst ( S / C=5×10 5 ). Replace with hydrogen three times, pressurize to 80bar with hydrogen, heat up to 100°C for 3h, cool down, release the pressure, open the kettle, GC analysis reaction conversion rate is greater than 99%, vacuum distillation to get 50g (S)-NAA, yield 95%, HPLC analysis ee value is 91%.
[0058] The structural formula of (S)-NAA is as follows:
[0059]
[0060] HPLC (OJ-H, n-hexane / i-PrOH=98 / 2, 1.0ml / min, 254nm, 40°C):t R (minor)=3.9min,t R (major) = 4.3min. 1 H NMR (400MHz, CDCl 3 ):7.02(dd,J=7.6,15.2Hz,2H),6.89(t,J=7.6Hz,1H),3.36-3.40(m,6H),2.67(q,J=7.6Hz,2H),2.31 (s, 3H), 1.25 (t, J = 7.6Hz, 3H), δ = 1.20 (d, J = 5.6Hz, 3H) ppm.
Embodiment 3
[0062] Other conditions are the same as in Example 2, the reaction pressure is 100 bar, the GC analysis reaction conversion rate is greater than 99%, and the HPLC analysis ee value is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com