Method for synthesizing dibenzophenazine by utilizing 2-naphthylamine under the effect of catalyst
A dibenzophenazine and catalyst technology, which is applied in the field of catalyzing the synthesis of dibenzophenazine from 2-naphthylamine, can solve the problems of easy explosion, environmental pollution, and low yield in the production process, and achieve safe and reliable production and simple process , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
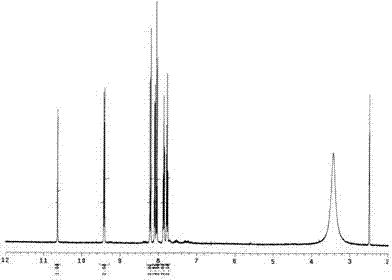
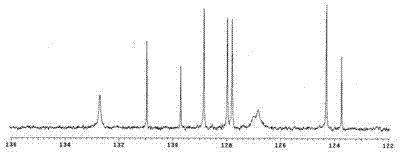
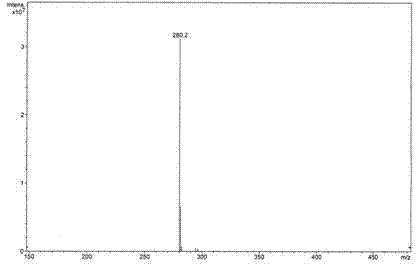
Image
Examples
Embodiment 1
[0020] Example 1: Weigh 0.18g (1mmol) of 2-naphthylamine hydrochloride and 0.06g (0.1mmol) of six-membered semicucurbitan HemiQ[6], respectively, and add them to 100mL of 0.1M hydrochloric acid aqueous solution. The reaction was carried out for 30 minutes under magnetic stirring. Naturally cool to room temperature and then filter with suction, recover the catalyst from the filtrate, rinse the precipitate with distilled water 4 to 5 times, and take it out after vacuum drying for 24 hours to obtain dibenzo[a, j]phenazine hydrochloride with a yield of about 47~ 56%.
Embodiment 2
[0021] Example 2: Weigh 0.18g (1mmol) of 2-naphthylamine hydrochloride and 0.12g (0.1mmol) of twelve-membered semicucurbitan HemiQ[6], respectively, and add them to 100mL of 0.1M hydrochloric acid aqueous solution, at 80°C The reaction was carried out for 30 minutes under magnetic stirring. Naturally cool to room temperature and then filter with suction, recover the catalyst from the filtrate, rinse the precipitate with methanol until it turns white, keep the rinse solution, spin the rinse solution, and recover the methanol, after the rinse solution is rotary evaporated, dry it in vacuum for 24 hours and take it out. That is, dibenzo[a, j]phenazine hydrochloride was obtained with a yield of about 52% to 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com