Method for preparing pentazocine intermediate
A technology of pentazocine and intermediates, which is applied in the field of drug synthesis, can solve the problems of difficult industrial production and low yield, and achieve the effects of short synthesis route, simple operation and high safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of Compound II
[0057]
[0058] In a 1L three-neck flask, add compound I (53g, 1.0eq), acetone 500mL, K 2 CO 3 (63g, 1.0eq), stirred at room temperature for 10-15min, added dropwise CH 3 I (64.8g, 1.0eq), no obvious exotherm, after dropping, add water (16.4g, 2.0eq) and react overnight at room temperature.
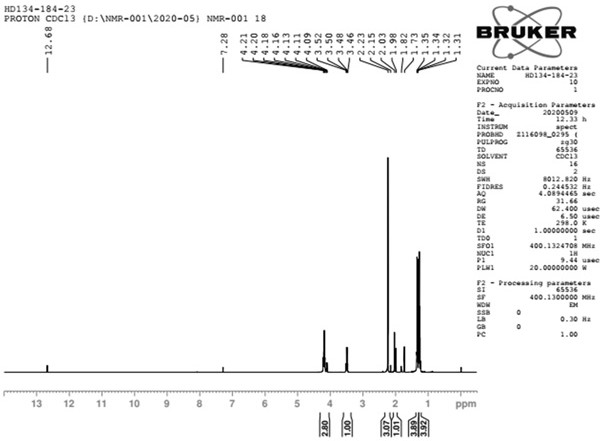
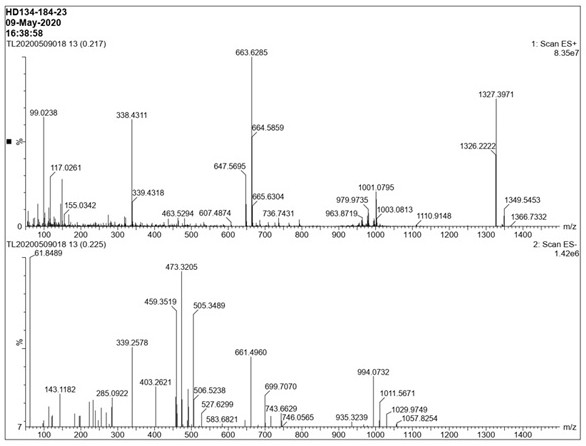
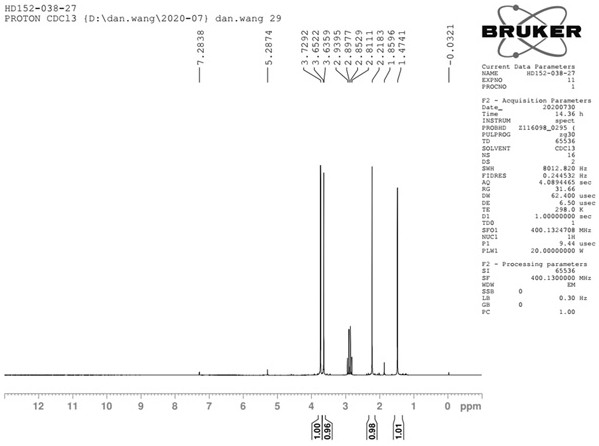
[0059] Post-treatment: Suction filtration, vacuum spin-drying of the filtrate, adding 100mL of water, 200mL of methyl tert-butyl ether (MTBE), stirring, liquid separation, extraction of the aqueous phase with 100mL of methyl tert-butyl ether (MTBE) once, combined organic phase, washed once with water, washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, and spin-dried to obtain 53g of crude compound II, which was distilled under reduced pressure (oil pump), with an external temperature of 50°C and a top temperature of 34-36°C to obtain 40.6 g of compound II, yield 76%. Compound II 1 H NMR spectrum see figure 1 , see th...
Embodiment 2
[0089] Synthesis of Compound II
[0090]
[0091] In a 100mL three-necked flask, add compound I (5g, 1.0eq), acetone 50mL, K2CO3 (6.37g, 1.2eq), stir at room temperature for 10min, add dimethyl sulfate (5.33g, 1.1eq) dropwise, without obvious exotherm, After dropping, the temperature was raised to reflux for 2 hours.
[0092] Post-processing: suction filtration, vacuum spin-drying of the filtrate, adding water and MTBE, stirring, liquid separation, extracting the aqueous phase once with MTBE, combining the organic phase, washing once with water, washing once with saturated sodium chloride, and drying over anhydrous sodium sulfate , spin-dried to obtain 2 g of the crude product of compound II, and the yield was 36%.
[0093] Synthesis of Compound III
[0094]
[0095] Potassium tert-butoxide (1eq), DMF (3V), add II (4g, 1eq) dissolved in DMF (2V) dropwise at about 0°C, add dropwise for 0.5h, keep warm for 0.5h, then add ethyl chloroacetate (1eq ), added dropwise for 0....
Embodiment 3
[0121] Add compound I (200g, 1.72 mol, 1.0eq), acetone 1500mL, K 2 CO 3 (238g, 1.72mol, 1.0eq), stirred at room temperature for 10-15min, added dropwise CH 3 I (244.5 g, 1.72 mol, 1.0 eq), CH was added dropwise 3 During the I process, there was no obvious exothermic reaction, and the reaction was carried out overnight at room temperature after the drop was completed. Detected by gas chromatography, it was found that the raw material compound I remained 19%, and the conversion of the raw material was incomplete. The temperature was raised to 35-40° C., and the reaction was continued for 3 hours, and detected by gas chromatography, it was found that 15% of the raw material compound I remained. Add 25g CH 3 I continued to react for 3 hours, and detected by gas chromatography, it was found that the raw material compound I still had about 15% remaining. Add 36g K 2 CO 3 And 400mL acetone, continue to react overnight. Next morning, by gas chromatographic detection, it was fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com