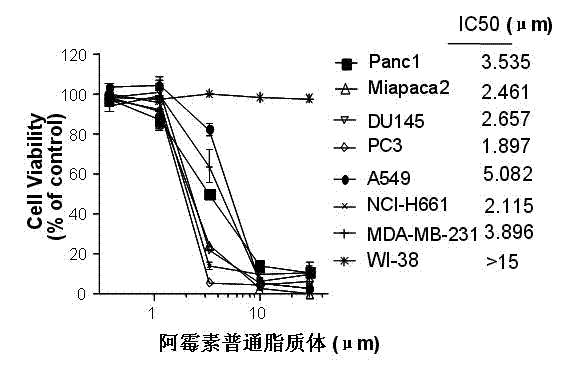
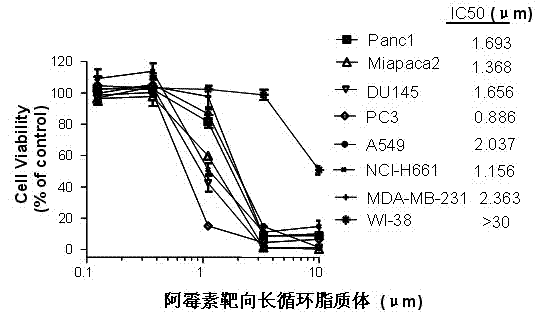
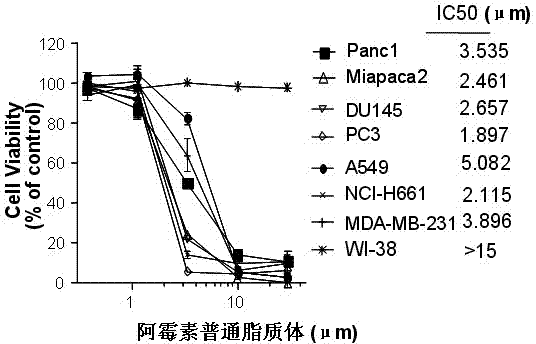
Polyethylene glycol-modified phospholipid derivative taking anilino-quinazoline as targeting ligand and preparation method thereof
A technology of anilinoquinazoline and anilinoquinazoline group, applied in polyethylene glycol modified phospholipid derivatives, the application field of new materials in anti-tumor drug preparations, to improve the effect of tumor treatment and drug efficacy , reduce the effect of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of polyethylene glycol 2000 bis-p-toluenesulfonate (TsO-PEG-TsO)
[0050] 1g HO-PEG 2000 -OH was dissolved in 20 mL of dichloromethane, then 0.35 g of triethylamine and 0.76 g of p-toluenesulfonyl chloride were added and stirred at room temperature for 10 hours. Using dichloromethane / ether (1:5) to recrystallize, the final TsO-PEG 2000 -TsO 0.89g, the yield is 90%.
[0051] 1 H-NMR (500MHZ, CDCl 3 )δ(ppm):2.47(s,6H,Ar-CH 3 ), 3.59 (m, multi H, back bone–OCH 2 CH 2 O-),4.19(t,4H,-CH 2 OTs),7.28(d,4H,J=7.9Hz,Ar-H),7.76(d,4H,J=7.9Hz,Ar-H).IR(KBr,cm -1 ):2885,1592,1463,1358,1266,1245
Embodiment 2
[0053] Preparation of polyethylene glycol 2000 bisphthalimide (phthalimide-PEG2000-phthalimide)
[0054] The TsO-PEG obtained in the previous step reaction 2000 - Dissolve TsO (0.7g) in 8mL DMF, add 0.65g phthaloyl potassium salt, reflux for 6h to stop the reaction, concentrate under reduced pressure to obtain light yellow oil, add 10mL dichloromethane, filter out insoluble matter, and the filtrate The crude product obtained by concentrating under reduced pressure was recrystallized with dichloromethane / diethyl ether (1:6) to obtain 0.55 g of the final product with a yield of 85%.
[0055] 1 H-NMR (500MHZ, CDCl 3 )δ (ppm): 3.61 (m, multi H, back bone–OCH 2 CH 2 O-),7.65-7.88(m,8H,Ar-H).IR(KBr,cm -1 ):2882,1715,1463,1356,1261.
Embodiment 3
[0057] Polyethylene glycol 2000 diethylamine (H 2 N-PEG 2000 -NH 2 ) preparation
[0058] The phthalimide-PEG obtained in the previous step reaction 2000 - phthalimide (0.6g) was dissolved in 10mL of absolute ethanol, 1.5mL of hydrazine hydrate was added, and the reaction was refluxed for 3h. Concentration under reduced pressure gave a yellow oil, which was recrystallized from dichloromethane / diethyl ether (1:10) to give 0.52 g of the final product, with a yield of 94%.
[0059] 1 H-NMR (500MHZ, CDCl 3 )δ(ppm):2.92(m,4H,-CH 2 NH 2 ),3.65(m,multi H,back bone–OCH 2 CH 2 O-).IR(KBr,cm -1 ): 3356, 2879, 1465, 1357, 1266.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com