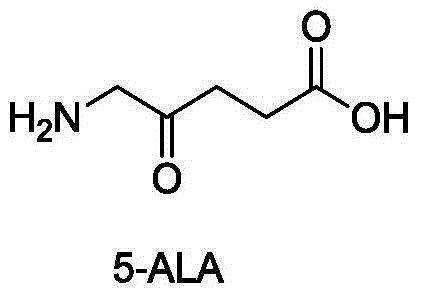
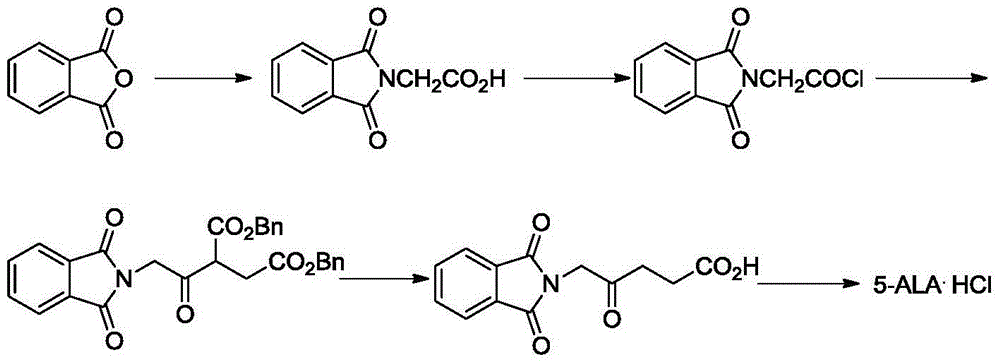
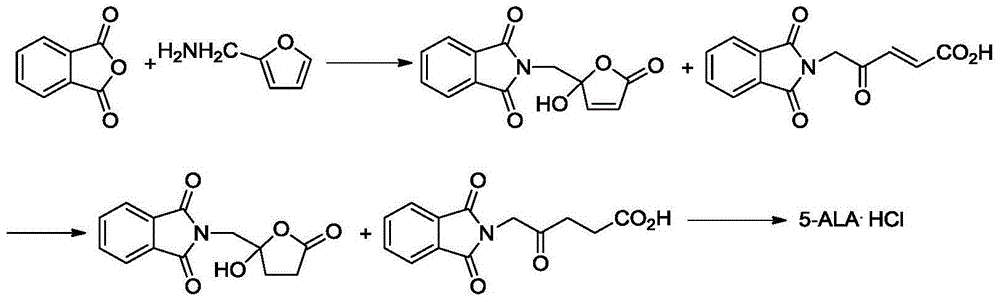
Preparation method of 5-aminolevulinic acid hydrochloride and intermediate of 5-aminolevulinic acid hydrochloride
A technology for aminolevulinic acid hydrochloride and intermediates, which is applied in the field of preparation of 5-aminolevulinic acid hydrochloride and its intermediates, can solve the problems of cumbersome operation steps and low yield, and achieve simple process, The effect of high yield and simplified operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 348g (3.0mol) of levulinic acid and 3500mL of methanol into a 5-liter four-necked flask, and add 480g (3.0mol) of liquid bromine dropwise under cooling in an ice-water bath (ie 0°C). Control the drop rate so that the temperature is between 0 and 10°C. After the dropwise addition, heat to reflux for 4-5 hours, and TLC detects that the reaction is complete. The reaction solution was cooled to room temperature and concentrated. The concentrated solution was poured into water and ethyl acetate, neutralized with sodium bicarbonate, and the organic phase was separated.
Embodiment 2
[0059] In a 5-liter four-necked bottle, add the ethyl acetate solution obtained in Example 1, add dropwise 200 mL of triethylamine, and detect by gas chromatography (GC detection) that after the content of methyl 3-bromolevulinate is lower than 1%, Add 2000 mL of DMF and 362 g (2.0 mol) of potassium phthalimide, and heat at 50°C for 1 hour. It was detected by TLC that the reaction was complete, cooled, filtered, and the filtrate was poured into ice water to precipitate a solid. Filter, wash, and dry to obtain 500 g of off-white solid with a purity of 95% as measured by HPLC.
Embodiment 3
[0061] In a 5-liter four-neck flask, add 500 g of the off-white solid obtained in Example 2, 3500 mL of 6N hydrochloric acid, heat to reflux, and react until the hydrolysis is complete. Cool, filter, decolorize the filtrate with activated carbon, and concentrate the aqueous phase to obtain an off-white solid. The mixed solution of methanol and ethyl acetate was recrystallized at 1:1 (volume ratio) to obtain 270 g of white crystals, with a yield of 53.7% (based on levulinic acid). mp: 149-152°C, 97% pure as measured by HPLC. The NMR data are as follows:
[0062] 1 HNMR (300M, D 2 o 2 )δ:2.50(2H,t,-CH 2 -),2.69(2H,t,-CH 2 -),3.92(2H,s,-CH 2 NH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com