Method for preparing mosapride citrate intermediate
A technology of mosapride citrate and intermediates, applied in the field of medicine, can solve the problems of high production difficulty, high cost, low purity of mosapride citrate and the like, and achieve the effects of reducing production costs and shortening routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
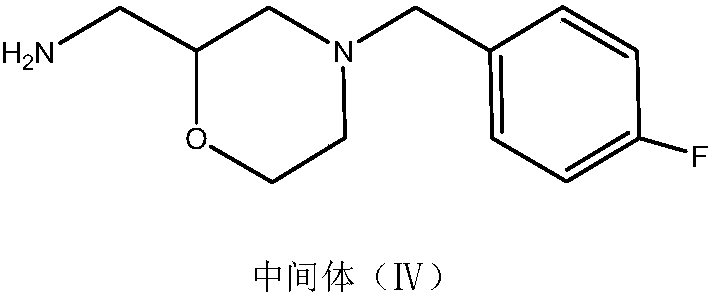
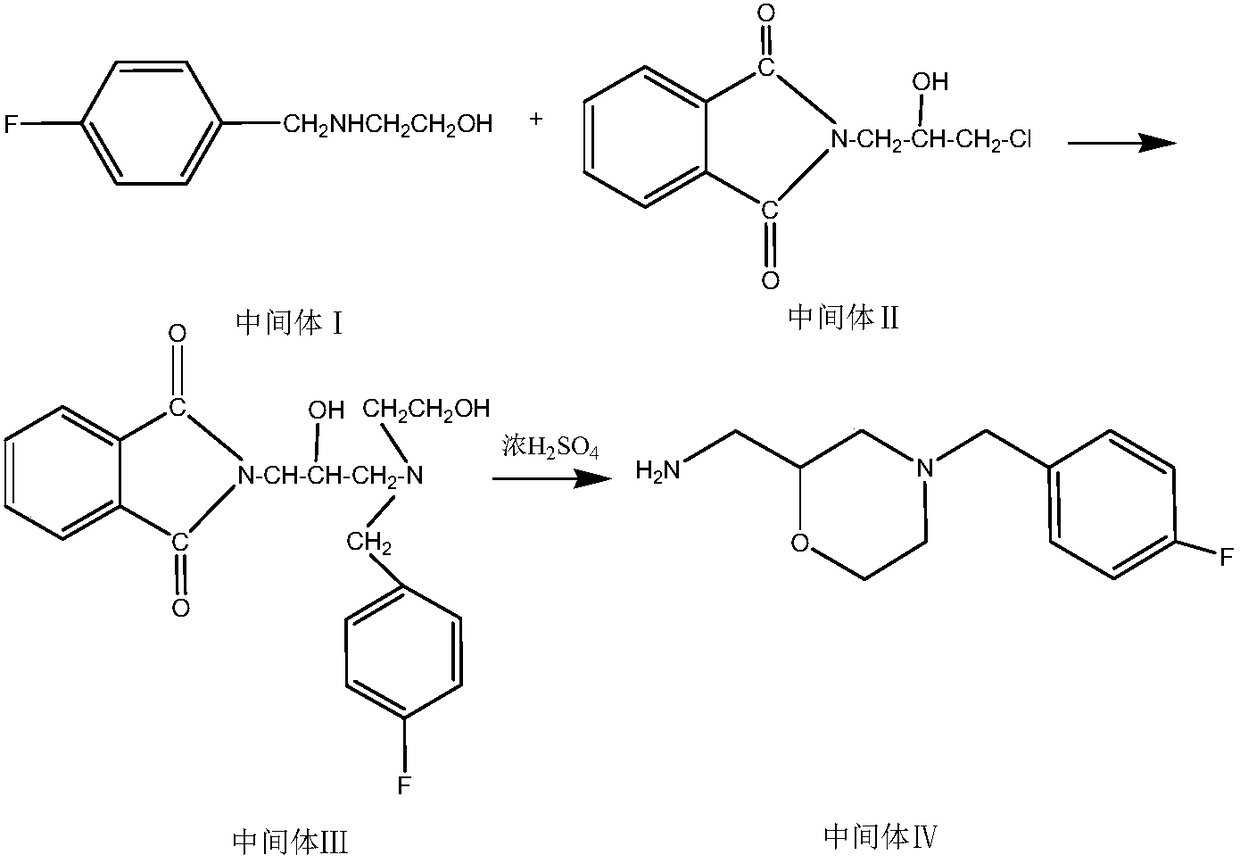
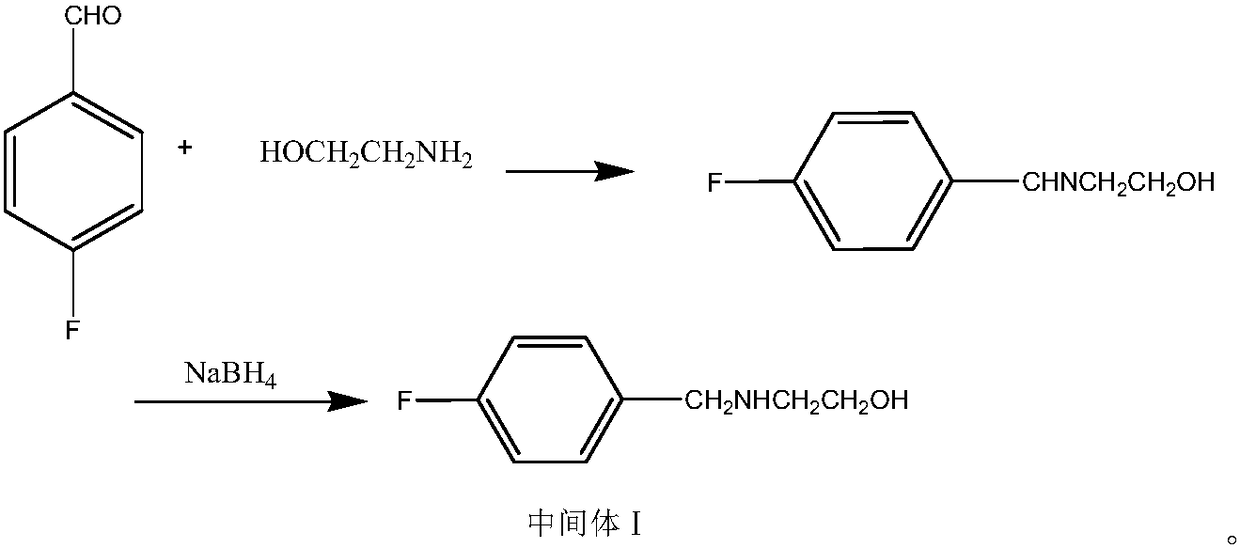
[0030] Preparation of intermediate N-3-[4-fluorobenzyl-2-(hydroxyl-ethylamine)-2-hydroxyisopropyl]phthalamide:
[0031] Add phthalimide potassium salt (33.4g, 0.18mol) and 166ml of absolute ethanol into a 500ml four-neck flask, heat to 60-70°C and stir to dissolve, add 0.216mol of dichloroisopropanol after the material is dissolved , 0.216mol triethylamine, heated and refluxed for 6 hours to generate the intermediate N-(2-hydroxy-3-chloropropyl) phthalimide, cooled to 50-60°C, and added dropwise the intermediate (30.5g , 0.18mol) ethanol solution of 2-(4-fluorobenzylamino)ethanol, keep warm for 3 hours, evaporate the ethanol to dryness under reduced pressure, add ethyl acetate to dissolve, filter with suction, wash the filtrate with water and salt, separate liquid, anhydrous sulfuric acid After drying the magnesium, filter it with suction and evaporate to dryness under reduced pressure to obtain a light yellow oily substance which is the intermediate N-3-[4-fluorobenzyl-2-(hyd...
Embodiment 2
[0033] Preparation of intermediate N-3-[4-fluorobenzyl-2-(hydroxyl-ethylamine)-2-hydroxyisopropyl]phthalamide:
[0034] Add phthalimide potassium salt (33.4g, 0.18mol) and 166ml of absolute ethanol into a 500ml four-neck flask, heat to 60-70°C and stir to dissolve, add 0.27mol of dichloroisopropanol after the material is dissolved , 0.27mol triethylamine, heated and refluxed for 6 hours to generate intermediate N-(2-hydroxyl-3-chloropropyl)phthalimide, cooled to 50-60°C, and added dropwise 0.216mol 2 -(4-Fluorobenzylamino)ethanol solution in ethanol, heat preservation reaction for 3 hours, evaporate the ethanol to dryness under reduced pressure, add ethyl acetate to dissolve, filter with suction, wash the filtrate with water and salt, separate liquid, dry over anhydrous magnesium sulfate, and pump Filter, evaporate to dryness under reduced pressure to obtain light yellow oil intermediate N-3-[4-fluorobenzyl-2-(hydroxyl-ethylamine)-2-hydroxyisopropyl]phthalamide (yield 83.6%, ...
Embodiment 3
[0036] Preparation of intermediate N-3-[4-fluorobenzyl-2-(hydroxyl-ethylamine)-2-hydroxyisopropyl]phthalamide:
[0037]Add phthalimide potassium salt (33.4g, 0.18mol) and 166ml of absolute ethanol into a 500ml four-neck flask, heat to 60-70°C and stir to dissolve, then add 0.3mol of dichloroisopropanol after the material is dissolved , 0.216mol triethylamine, heated and refluxed for 6 hours to generate the intermediate N-(2-hydroxy-3-chloropropyl) phthalimide, cooled to 50-60°C, and added dropwise the intermediate (30.5g , 0.18mol) ethanol solution of 2-(4-fluorobenzylamino)ethanol, keep warm for 3 hours, evaporate the ethanol to dryness under reduced pressure, add ethyl acetate to dissolve, filter with suction, wash the filtrate with water and salt, separate liquid, anhydrous sulfuric acid After magnesium drying, filter with suction and evaporate to dryness under reduced pressure to obtain light yellow oil intermediate N-3-[4-fluorobenzyl-2-(hydroxyl-ethylamine)-2-hydroxyisop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com