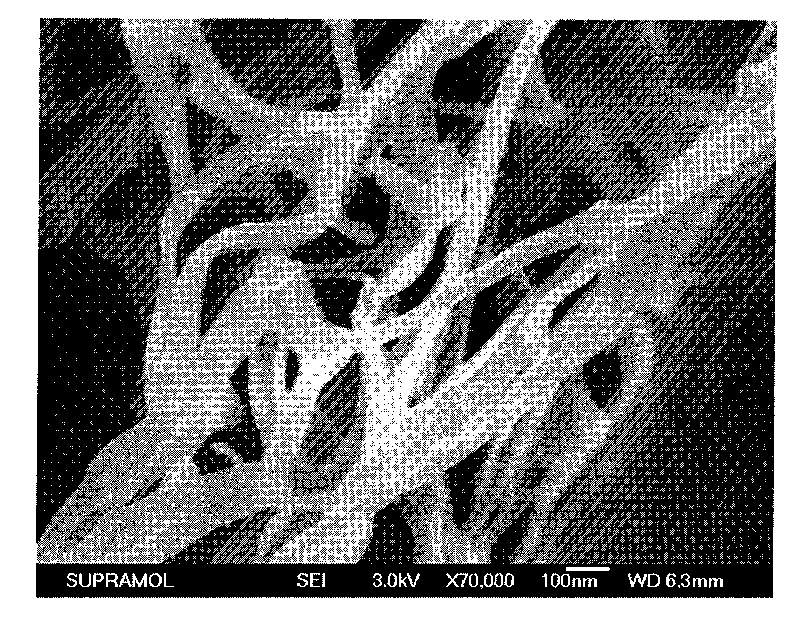
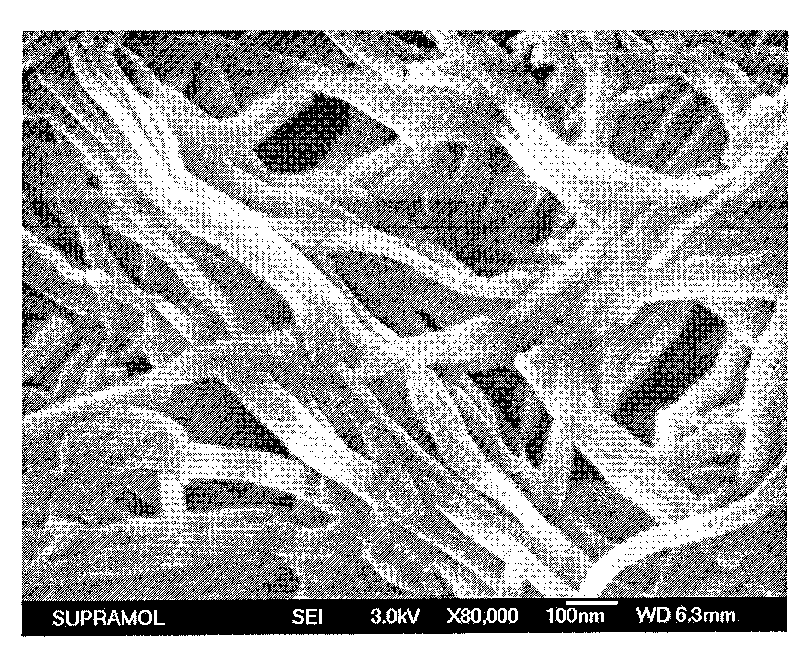
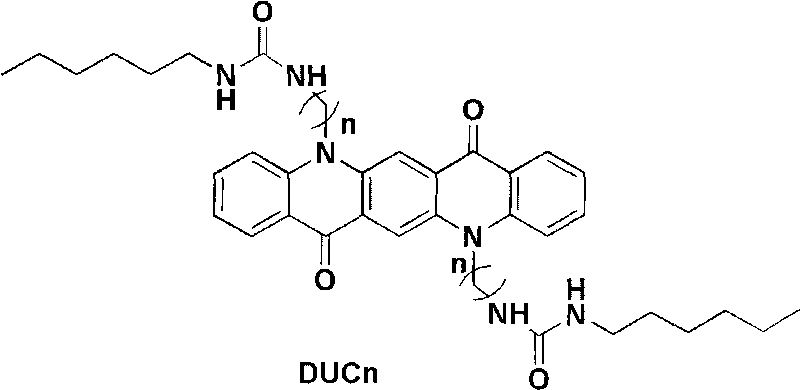
Quinacridone derivatives containing urea bonds and gels thereof
A technology of quinacridone and derivatives is applied in the field of quinacridone derivative luminescent materials and the gel formed thereof, and can solve rare problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of compound DUC6
[0025] Under nitrogen protection conditions, DCB6 (2.55 g), sodium iodide (0.5 g) and phthalimide potassium salt (2.96 g) in dimethylformamide solution (100 ml) were heated at 60 ° C for 24 hours, cooled , 200 ml of water was added to stop the reaction, and the resulting solid was subjected to column chromatography (silica gel, chloroform) to obtain 2.70 g (88%) of the orange DUP6 target product.
[0026] DUP6 (0.90 g) and hydrazine hydrate (5.0 ml) were refluxed in 90 ml of tetrahydrofuran / ethanol=1:1 (volume / volume) for 5 hours, the organic solvent was evaporated under reduced pressure, and 100 ml of water was added for suction filtration to obtain the filter cake to dry. The solid was recrystallized from chloroform and cyclohexane to obtain the target product DUN6 (0.54 g, 90%).
[0027] DUN6 (0.4 g) was added to a solution of 50 ml of hexyl isocyanate (0.24 g) in 30 ml of chloroform / methanol=1:1 (volume / volume), and s...
Embodiment 2
[0028] Embodiment 2: the synthesis of compound DUC8
[0029] The synthesis of compound DUC8 is the same as in Example 1, except that 1,8-dibromooctane is used instead of 1,6-dibromohexane when preparing DCBn. Product DUC8 mass spectrum molecular ion peak: 820.8. Elemental analysis according to chemical formula C 50 h 72 N 6 o 4 Calculated: C: 73.13%; H: 8.84%; N: 10.23%; O: 7.79%. Experimental values: C: 73.28%; H: 8.79%; N: 10.02%.
Embodiment 3
[0030] Embodiment 3: the synthesis of compound DUC10
[0031] The synthesis of compound DUC10 is the same as in Example 1, except that 1,10-dibromodecane is used instead of 1,6-dibromohexane when preparing DCBn. Product DUC10 mass spectrum molecular ion peak: 877.2. Elemental analysis according to chemical formula C 54 h 80 N 6 o 4 Calculated: C: 73.93%; H: 9.19%; N: 9.58%; O: 7.30%. Experimental values: C: 73.69%; H: 9.35%; N: 9.40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com