Preparation method of 7-methoxy-1-naphthylethylamine
A technology of methoxyl and naphthylethylamine is applied in the field of preparation of key intermediate 7-methoxy-1-naphthaleneethylamine, and achieves the effects of avoiding high temperature and high pressure reaction, low cost of raw materials and reducing environmental pollution problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
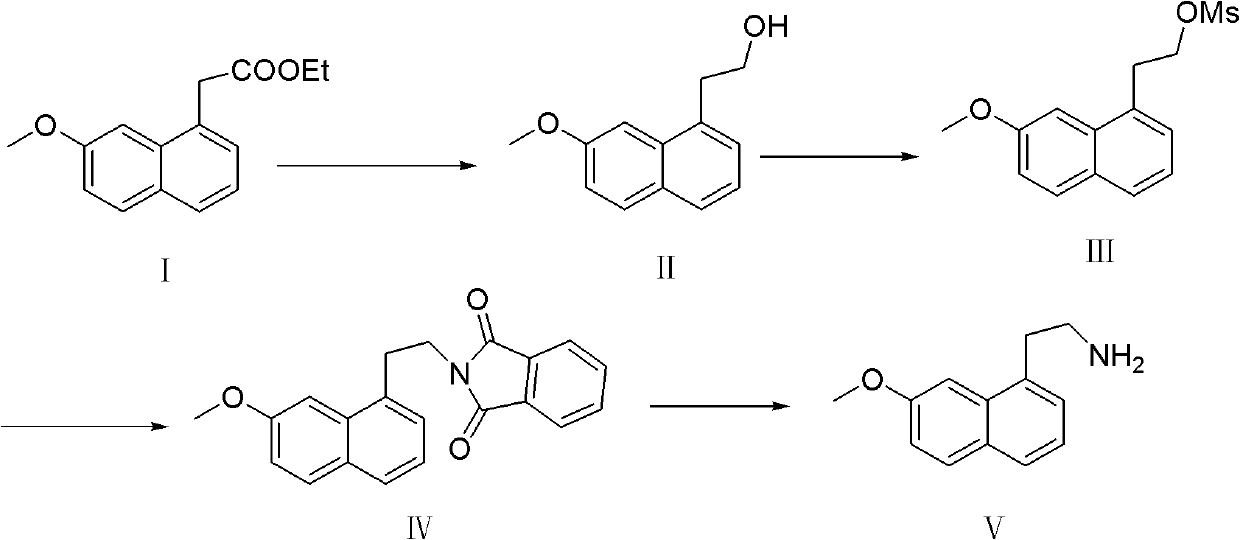
[0022] A preparation method of 7-methoxy-1-naphthylethylamine, the steps are as follows:
[0023] 1, the preparation of 7-methoxy-1-naphthyl alcohol
[0024] Dissolve 24g of ethyl 7-methoxy-1-naphthaleneacetate in a mixture of 50mL of water and 80mL of ethanol, stir mechanically, add 13.3g of sodium borohydride, and reflux for 2 hours. TLC shows that the reaction is complete, cool to room temperature, and add 200mL of water, extracted twice from water with 300mL ethyl acetate respectively, combined ethyl acetate, washed once with water, once with saturated ammonium chloride solution, once with saturated brine, and spin-dried to obtain 15g of yellow solid. It was confirmed to be 7-methoxy-1-naphthyl alcohol with a melting point of 82-84°C and a yield of 74%.
[0025] 2, Preparation of 7-methoxyl-1-naphthyl ethyl methanesulfonate
[0026] Add 11g of 7-methoxy-1-naphthyl alcohol and 11g of triethylamine obtained in step 1 into a 250mL three-necked flask, add 80mL of dichloromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com