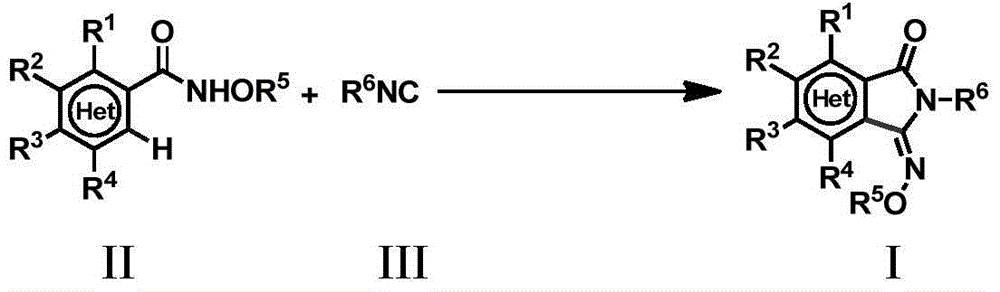
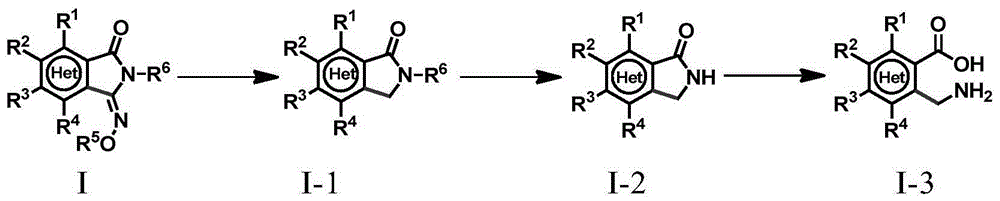
Method for preparing 3-imino isoindoline ketone compounds
A compound, aryl technology, applied in the field of organic synthesis, can solve the problems of limited application, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
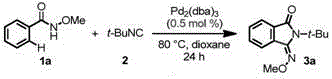
[0094] Example 1 Trans-2-tert-butyl-3-methoxyiminoisoindolinone (3a)
[0095]
[0096] Under air (air) (1atm), add amide compound 1a15.1mg (0.10mmol), tert-butylisonitrile 18μL, (0.15mmol), Pd in a 15mL reaction tube 2 (dba) 32.3mg (0.0025mmol), 1mL of 1,4-dioxane (dioxane), mix well, and react at 80°C for 0.5 hours. After the reaction was completed, it was cooled to room temperature and concentrated to obtain a crude product. The crude product was separated with a preparative plate (the volume ratio of petroleum ether to ethyl acetate was 20:1), and 21.7 mg of the product trans-2-tert-butyl-3-methoxyiminooxyisoindolinone (94% ); 1 HNMR (400MHz, CDCl 3 )δ8.26–8.24(m,1H),7.77–7.75(m,1H),7.58–7.50(m,2H),4.06(s,3H),1.76(s,9H); 13 CNMR (100MHz, CDCl 3 )δ167.33, 149.98, 132.78, 131.74, 131.10, 128.53, 127.73, 122.65, 63.24, 58.65, 29.60; HRMS (ESI-TOF) m / zCalcdforC 13 h 17 N 2 o 2 (M+H) + :233.1285,found:233.1276.
Embodiment 2
[0097] Example 2 trans-7-methyl-2-tert-butyl-3-methoxyiminoisoindolinone (3b)
[0098]
[0099] The procedure was similar to that of Example 1, except that the corresponding starting compound 1b was used instead of compound 1a. Reaction time 10 hours, productive rate 67%; 1 HNMR (400MHz, CDCl 3 )δ8.13(d, J=7.6Hz, 1H), 7.43(t, J=7.6Hz, 1H), 7.28(d, J=7.6Hz, 1H), 4.05(s, 3H), 2.67(s, 3H), 1.75(s, 9H); 13 CNMR (100MHz, CDCl 3 )δ168.37, 149.80, 137.14, 133.71, 132.35, 129.10, 128.40, 125.51, 63.30, 58.58, 29.73, 17.90; HRMS (ESI-TOF) m / zCalcdforC 14 h 19 N 2 o 2 (M+H) + :247.1441,found:247.1451.
Embodiment 3
[0100] Example 3 Trans-6-methyl-2-tert-butyl-3-methoxyiminoisoindolinone (3c)
[0101]
[0102] The procedure was similar to that of Example 1, except that the corresponding starting compound 1c was used instead of compound 1a. Reaction time 0.5 hour, productive rate 90%; 1 HNMR (400MHz, CDCl 3 )δ8.11(d,J=7.6Hz,1H),7.55(s,1H),7.36(dd,J=8.0,0.8Hz,1H),4.05(s,3H),2.44(s,3H), 1.75(s,9H); 13 CNMR (100MHz, CDCl 3 )δ167.63, 150.25, 141.86, 133.63, 132.12, 127.58, 126.18, 123.09, 63.23, 58.66, 29.70, 21.81; HRMS (ESI-TOF) m / zCalcdforC 14 h 19 N 2 o 2 (M+H) + :247.1441,found:247.1452.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com