Fluorescence sensor for rhodamine B as well as preparation and application thereof
A fluorescent sensor and reaction technology, applied in the field of biosensors, can solve the problems of large detection limit, low sensitivity, and inaccurate detection of Fe, etc., and achieve the effects of small detection limit, good sensitivity, and excellent specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
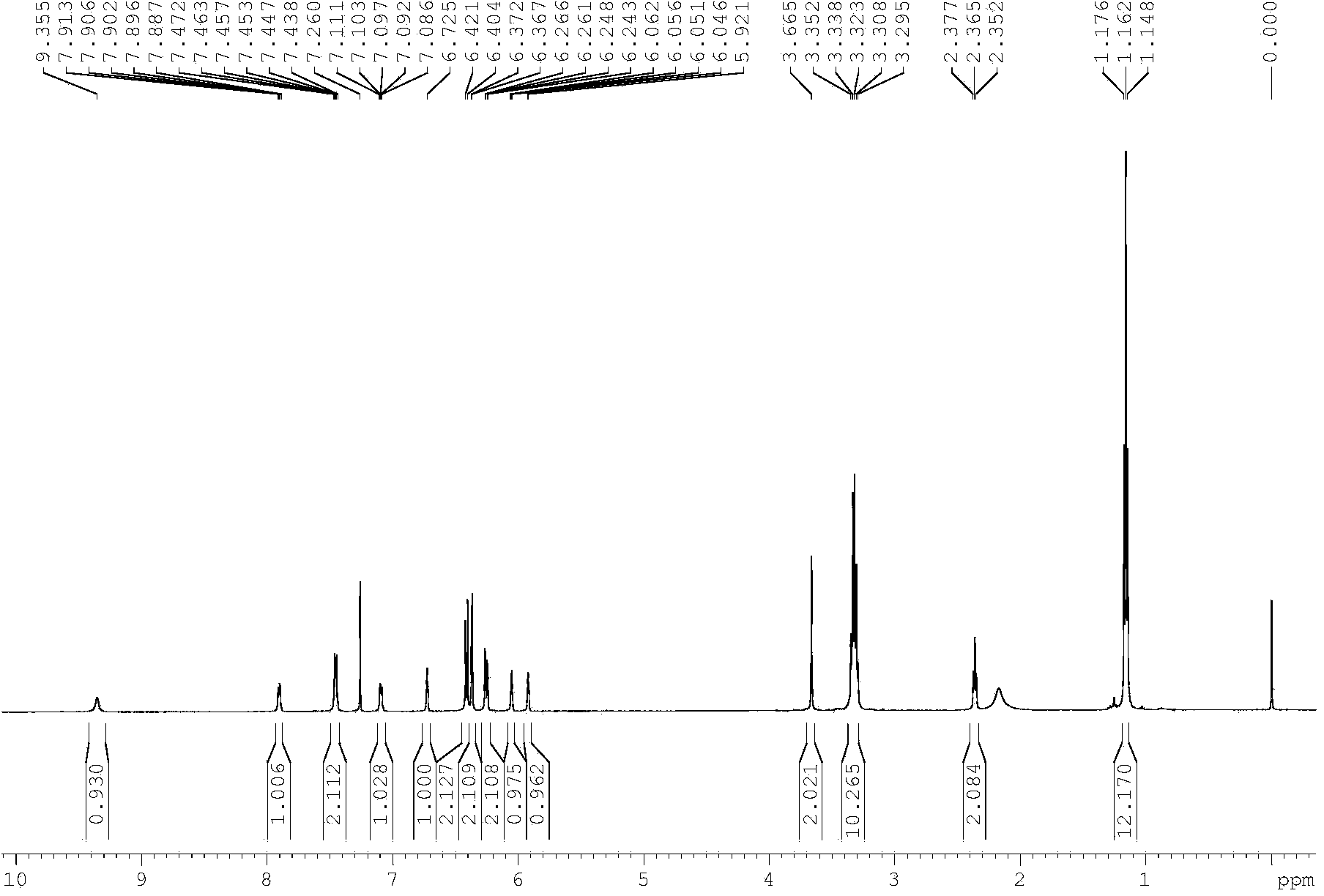
[0034] Synthesis of embodiment 1 sensor compound
[0035] 1, the synthetic method of compound 3
[0036] Weigh 500mg of compound 2 (1.13mmol) into a 25ml round bottom flask, add about 5ml of absolute ethanol, stir to dissolve, add 754μl (11.3mmol) of ethylenediamine dropwise to the flask, and heat to reflux. During the reaction process, the progress of the reaction was detected by TLC. After 12 hours of complete reaction, the mixture was cooled to room temperature, and the mixture was separated to obtain about 510 mg of pure compound 3.
[0037] 2. Synthesis of target compound 1
[0038]Weigh 200mg of compound 3 (0.413mmol) obtained in step 1 and dissolve it in about 5ml of methanol, then add 118mg (1.24mmol) of pyrrole-2-carbaldehyde, stir at room temperature, and use TLC to detect the reaction progress during the reaction, 12 The reaction is complete within 1 hour, then weigh 175 mg of sodium triacetoxyborohydride and add it to the reaction, and continue the reaction for 3...
Embodiment 2
[0039] Embodiment 2 UV-Vis absorption performance test
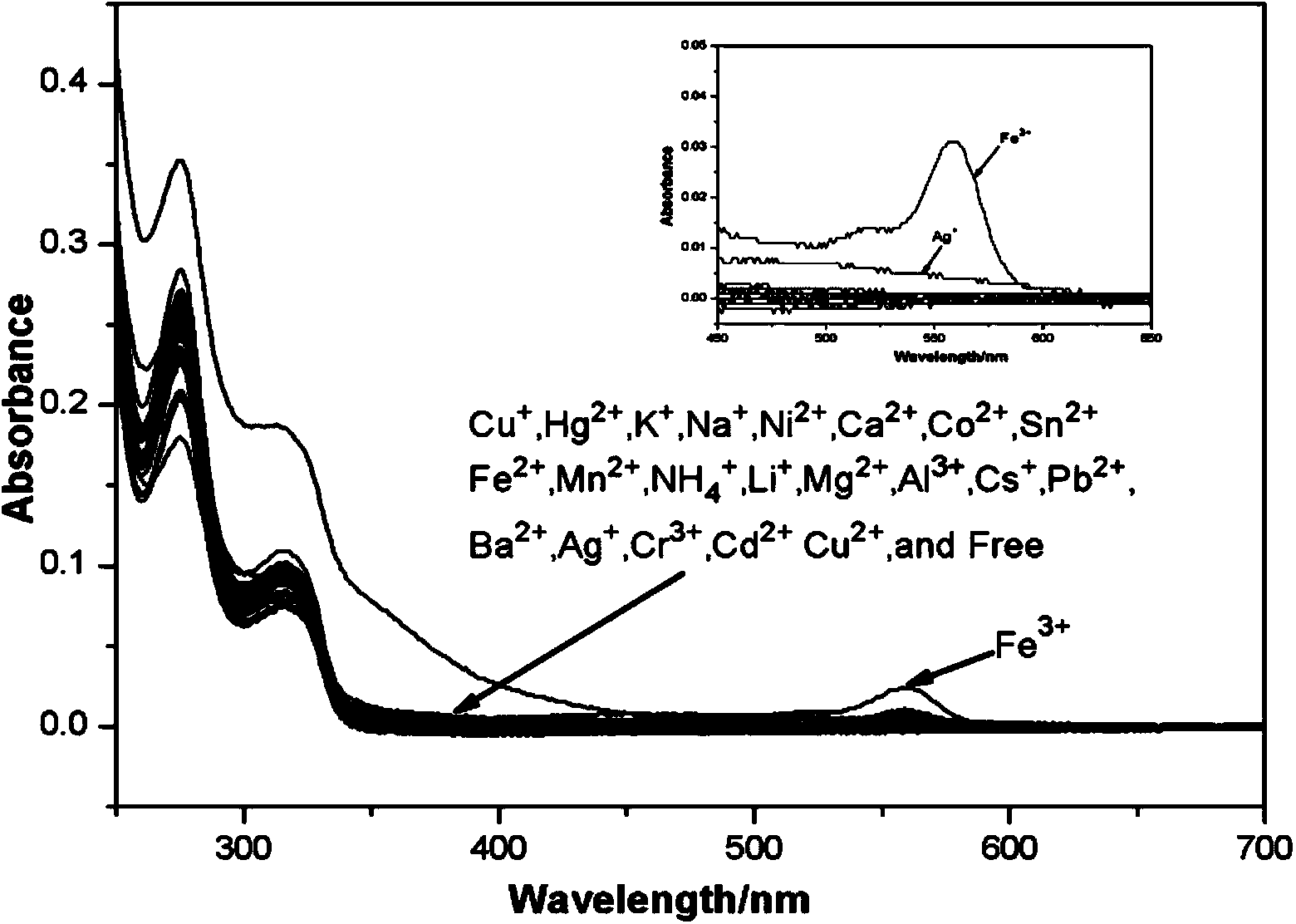
[0040] 1. UV absorption performance test for different heavy metal ions
[0041] Compound 1 was prepared into a 10 μmol / L methanol-water mixed solution (3:2, v:v), and 3000 μL of the mixed solution was placed in a liquid pool to detect the ultraviolet absorption value. Use a pipette gun to measure the prepared CdCl 2 2.5H 2 O, CuCl 2 2H 2 O,AlCl 3 ,KCl,FeCl 3 ·6H 2 O,PbCl 2 ,SnCl 2 ·H 2 O, AgNO 3 ,HgCl 2 ,NiCl 2 ·6H 2 O,FeCl 2 4H 2 O,MgCl 2 ·6H 2 O, NaCl, ZnCl 2 ,CrCl 3 ·6H 2 O,Ba(NO 3 ) 2 , CuCl, LiCl·H 2 O,MnCl 2 4H 2 O,CoCl 2 ·6H 2 O,CaCl 2 ,NH 4 Cl, the plasma solution was added to the liquid pool, so that the concentration ratio of compound 1 and ions was 1:5, and after 10 minutes, the changes in the ultraviolet absorption spectrum were observed and recorded. The absorption spectrum is as figure 2 As shown, it was found that adding FeCl 3 ·6H 2 After O, the UV absorption value has a...
Embodiment 3
[0042] Embodiment 3 Fluorescence performance test
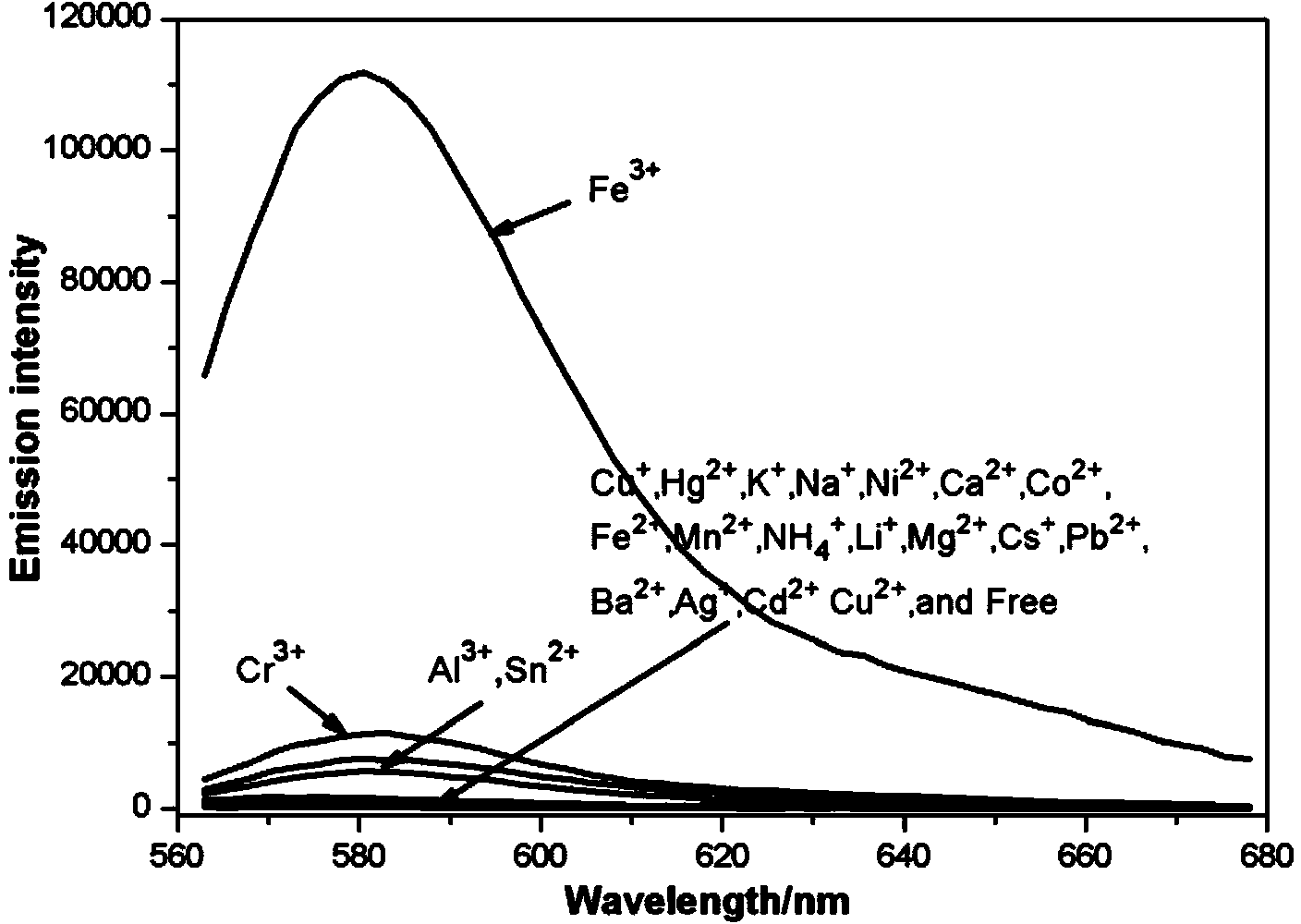
[0043] 1. Fluorescence response test to different heavy metal ions
[0044] Compound 1 was prepared into a 10 μmol / L methanol-water mixed solution (3:2, v:v), and 3000 μL was placed in a liquid pool to detect its initial fluorescence value. Measure the prepared CdCl with a pipette gun 2 2.5H 2 O, CuCl 2 2H 2 O,AlCl 3 ,KCl,FeCl 3 ·6H 2 O,PbCl 2 ,SnCl 2 ·H 2 O, AgNO 3 ,HgCl 2 ,NiCl 2 ·6H 2 O,FeCl 2 4H 2 O,MgCl 2 ·6H 2 O, NaCl, ZnCl 2 ,CrCl 3 ·6H 2 O,Ba(NO 3 ) 2 , CuCl, LiCl·H 2 O,MnCl 2 4H 2 O,CoCl 2 ·6H 2 O,CaCl 2 ,NH 4 Cl, the plasma solution was added to the liquid cell, so that the molar ratio of compound 1 and ions in the solution was 1:5, and the changes in the fluorescence spectrum were observed and recorded. Fluorescence spectrum such as image 3 As shown, it was found that when FeCl was added 3 ·6H 2 After O, the fluorescence value at 581nm changes significantly, which shows that the fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com