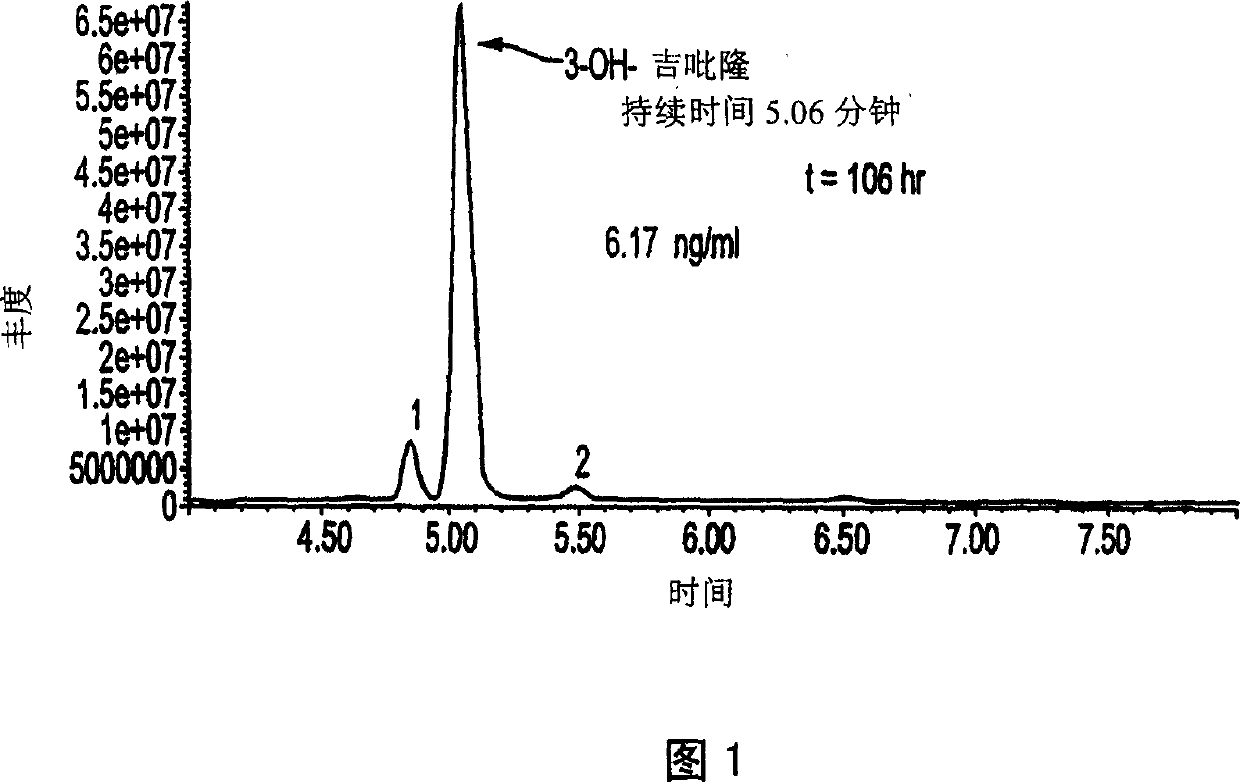
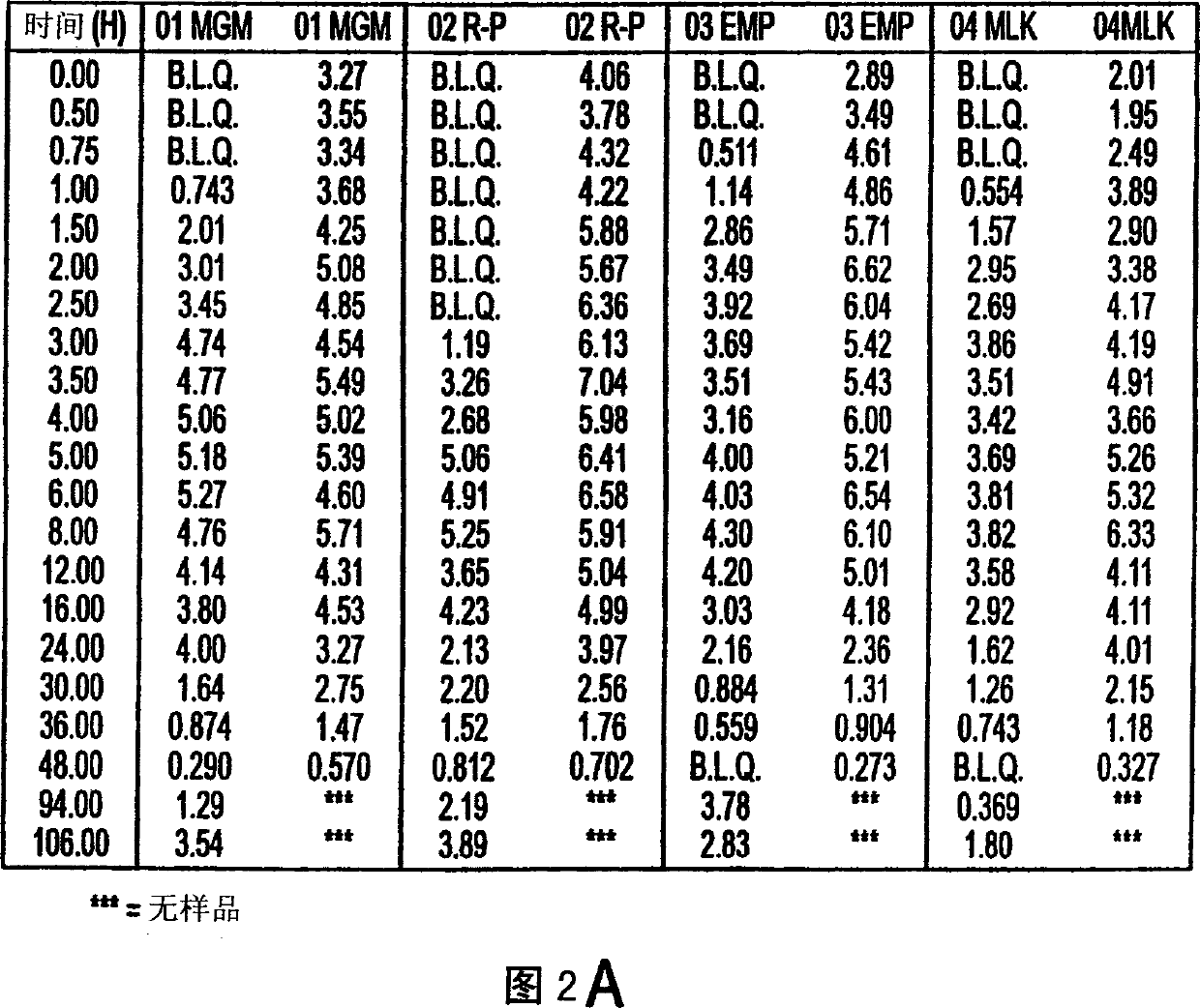
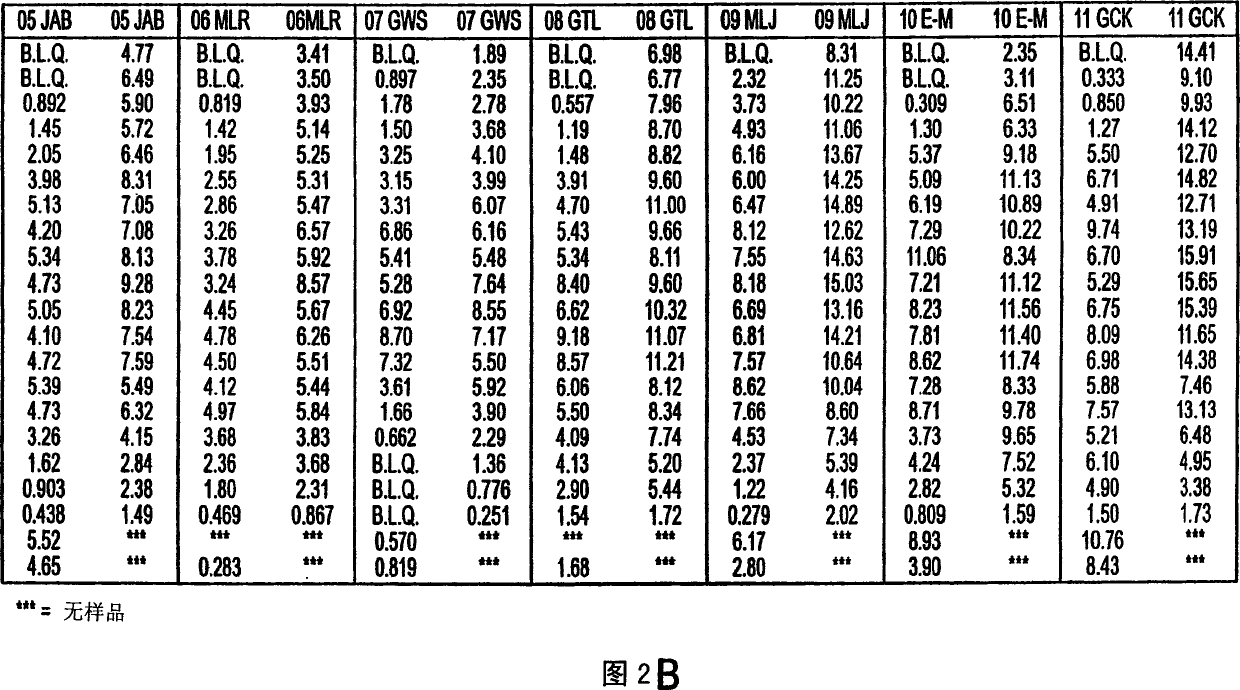
Use of bioactive metabolites of gepirone for treatment of psychological disorders
A technology of biological activity and metabolites, which is applied in the field of using gepirone bioactive metabolites to treat psychological disorders, and can solve problems such as no biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The methods of use and preparation of the compounds making up the present invention will become clearer in the following examples. These examples are for the purpose of illustrating the invention and not limiting the invention thereto, and all references cited in this specification for any purpose can be utilized and believed and can be used as specific examples of the present invention. Example. All references cited are incorporated herein by reference. Embodiment 1: Preparation of 3-OH gepirone (I) A. two-4-nitrobenzyl peroxydicarbonate (III) two-4-nitrobenzyl peroxydicarbonate can utilize (Strain et al., "Japan American Chemical Science", 1950, 72:1254; incorporated herein by reference). Thus, a cold solution of 4-nitrobenzyl chloroformate (10.11 g, 4.7 mmol) in acetone (20 mL) was added dropwise over a period of 30 minutes from 30% H 2 o 2 (2.7mL, 24mmol) and 2.35 N NaOH (20mL, 47mmol) in a cold mixture. The mixture was stirred well for 15 minutes, then filtere...
Embodiment 2
[0040] Experimental results: C, 60.21; H, 7.79; N, 18.32 Example 2: Comparison of 3-OH gepirone, gepirone metabolites and gepirone
[0041] compound
[0042] The short half-life of gepirone is attributed to its high oil solubility, which makes it more susceptible to first-pass degradation in the liver. Because 3-OH gepirone has little solubility in esters, its first-pass degradation profile results in a long half-life in plasma. Furthermore, the ester solubility range of 3-OH gepirone (approximately 5:1 to 8:1) is generally within the acceptable range for psychoactive drugs acting on brain receptors, while Broto's calculation method is not considered because of high standard deviation. within range. Therefore, 3-OH gepirone has excellent properties from the point of view of an immediate acting pharmacological compound avoiding first pass degradation in the liver. Embodiment 3: 3-OH gepirone preparation
Embodiment 3
[0043] The 3-OH gepirone compositions and formulations of the present invention are designed to administer to mammals, preferably humans, an effective amount of an anxiolytic, antidepressant, psychotropic 3-OH gepirone, or a pharmacologically acceptable form thereof. its salts. Effective formulations of about 0.01 to 40 mg / kg body weight are envisioned, with a preferred range of about 0.1-0.2 mg / kg body weight. For some central nervous disorders, it is recommended to take 15-90mg per day, preferably 30-60mg per day. (See US Patent 4,771,053 to Cott et al., incorporated herein by reference). The administration of the biologically active gepirone metabolite according to the present invention can be through injection, oral administration, buccal administration, rectal administration, external application, etc., but oral administration is preferred. For alleviating severe depression, the clinical dose range is less than 100 mg / day, generally 15-90 mg / day, preferably 30-60 mg / day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com