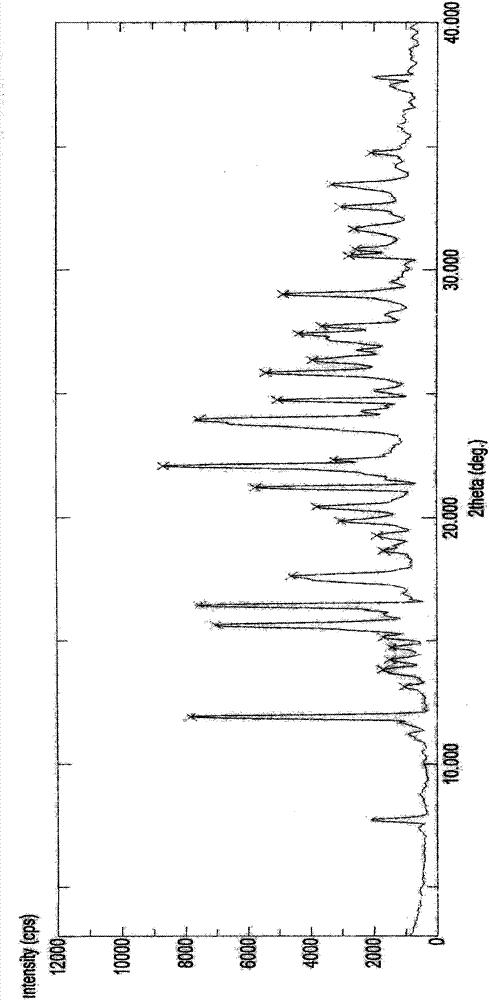
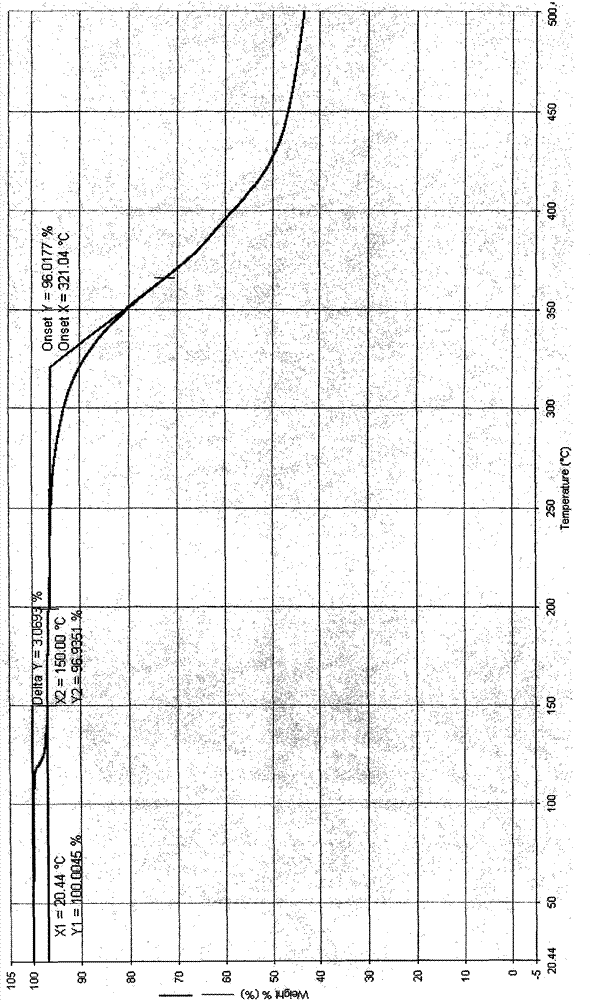
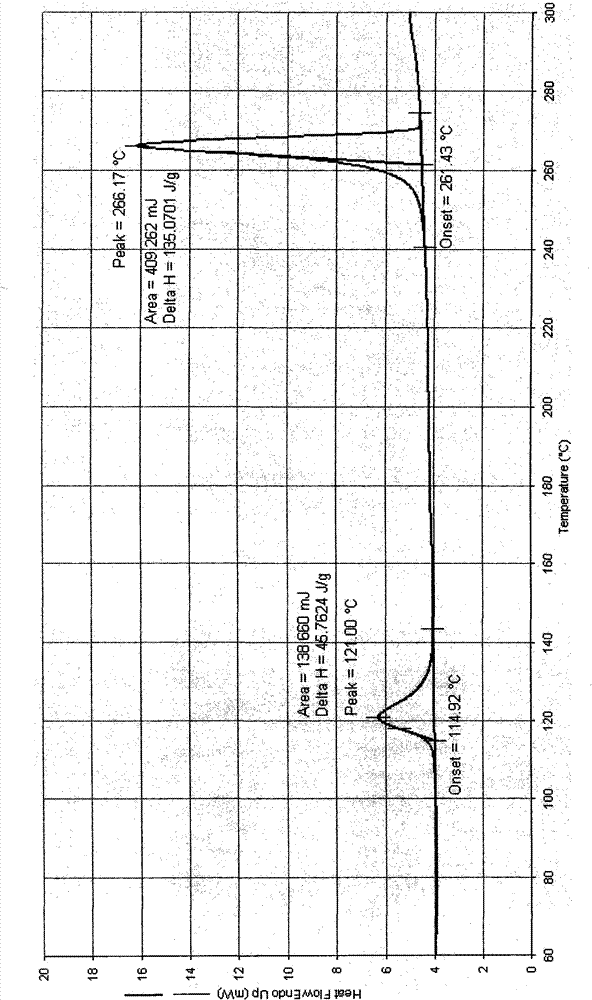
Crystal IV of 3-(substituted dihydroisoindolinone-2-yl)-2,6-piperidinediketone and medicinal composite thereof
A technology of isoindole and composition, applied in the preparation method of the crystal and its pharmaceutical composition, 3-piperidine-2 field, can solve the problems of unfavorable industrial scale stable parallel production preparation and no consideration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0308] Preparation of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione IV crystals
[0309] 5 grams of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione were dissolved in 350ml of acetonitrile at 60°C under stirring In, add activated carbon 0.25 gram, filter after stirring for ten minutes. The filtrate was cooled to room temperature at a rate gradient of 5° C. every 2 hours with stirring, and then left to stand. The solvent is slowly volatilized at around 20°C for a long time until the crystals are precipitated. The solid was collected by filtration and evaporated to constant weight at room temperature. Weighing: 2.1 g; Yield: 42%.
[0310]
Embodiment 2
[0311] The prescription and preparation technology of embodiment 2 tablet:
[0312] The above-mentioned 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione was prepared with several excipients as follows Crystalline IV is formulated as tablets containing 10 mg each.
[0313] Prescription status:
[0314]
[0315] Preparation process: the preparation method of IV tablets containing 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione crystals is The above-mentioned excipients and 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione crystals were increased in equal amounts Mix evenly, add 10% PVP solution as an appropriate amount of binder, make a soft material, sieve and granulate, dry the wet granules, sieve and granulate, add magnesium stearate, mix evenly, and compress into tablets. See Table 6 for the test results of cumulative dissolution of tablet prescription 1, and see Table 6 for the dissolution curve of tablet prescript...
Embodiment 3
[0320] The prescription and preparation technology of embodiment 3 capsules:
[0321] 3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione crystals were prepared with several excipients as follows IV formulated as capsules containing 10 mg each.
[0322]
[0323] Preparation process: the manufacture method of IV capsules containing 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione crystals is to Mix the above-mentioned excipients with 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione crystals, add 10% The PVP solution is made into wet granules, dried, sieved for granulation, added with magnesium stearate, mixed evenly, and packed into capsules. Or without granulation, mix 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione crystals with the above excipients Mix evenly, sieve, and directly pack into capsules. See Table 8 for the test results of cumulative dissolution of capsule prescription 1, and see Table 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com